| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,2,2-Trichloroethane-1,1-diol | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1698497 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.562 | ||
| EC Number |
| ||
| 101369 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2811 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties[3] | |||
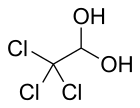
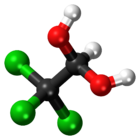
| CCl3CH(OH)2 | |||
| Molar mass | 165.39 g·mol−1 | ||
| Appearance | Colorless solid | ||
| Odor | Aromatic, slightly acrid | ||
| Density | 1.9081 g/cm3 | ||
| Melting point | 57 °C (135 °F; 330 K) | ||
| Boiling point | 98 °C (208 °F; 371 K) (decomposes) | ||
| 660 g/(100 ml) | |||
| Solubility | Very soluble in benzene, ethyl ether, ethanol | ||
| log P | 0.99 | ||
| Acidity (pKa) | 9.66, 11.0[2] | ||
| Structure | |||
| Monoclinic | |||
| Pharmacology | |||
| N05CC01 (WHO) | |||
| Oral syrup, rectal suppository | |||
| Pharmacokinetics: | |||
| Well absorbed | |||
| Hepatic and renal (converted to trichloroethanol) | |||
| 8–10 hours | |||
| Bile, feces, urine (various metabolites not unchanged) | |||
| Legal status |
| ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H301, H315, H319 | |||
| P264, P270, P280, P301+P310, P302+P352, P305+P351+P338, P321, P330, P332+P313, P337+P313, P362, P405, P501 | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1100 mg/kg (oral) | ||
| Safety data sheet (SDS) | External MSDS[dead link] | ||
| Related compounds | |||
Related compounds
|
Chloral, chlorobutanol, Triclofos | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Chloral hydrate is a geminal diol with the formula Cl3C−CH(OH)2. It was first used as a sedative and hypnotic in Germany in the 1870s. Over time it was replaced by safer and more effective alternatives but it remained in usage in the United States until at least the 1970s.[4] It sometimes finds usage as a laboratory chemical reagent and precursor. It is derived from chloral (trichloroacetaldehyde) by the addition of one equivalent of water.
- ^ Vardanyan, R.S.; Hruby, V.J. (2006). "Soporific Agents (Hypnotics and Sedative Drugs)". Synthesis of Essential Drugs. pp. 57–68. doi:10.1016/B978-044452166-8/50004-2. ISBN 978-0-444-52166-8.
- ^ Gawron, O.; Draus, F. (1958). "Kinetic Evidence for Reaction of Chloralate Ion with p-Nitrophenyl Acetate in Aqueous Solution". Journal of the American Chemical Society. 80 (20): 5392–5394. doi:10.1021/ja01553a018.
- ^ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (85th ed.). CRC Press. pp. 3–98. ISBN 978-0-8493-0484-2.
- ^ Kales, Anthony (1 September 1970). "Hypnotic Drugs and Their Effectiveness: All-night EEG Studies of Insomniac Subjects". Archives of General Psychiatry. 23 (3): 226–232. doi:10.1001/archpsyc.1970.01750030034006. PMID 4318151.