| |

| |
| Names | |
|---|---|
| IUPAC name
Chlorite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.123.477 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
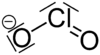
| ClO− 2 | |
| Molar mass | 67.452 |
| Conjugate acid | Chlorous acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The chlorite ion, or chlorine dioxide anion, is the halite with the chemical formula of ClO−
2. A chlorite (compound) is a compound that contains this group, with chlorine in the oxidation state of +3. Chlorites are also known as salts of chlorous acid.