You can help expand this article with text translated from the corresponding article in Turkish. (September 2024) Click [show] for important translation instructions.
|
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trichloromethane | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | R-20, TCM | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.603 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1888 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
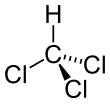
| CHCl3 | |||
| Molar mass | 119.37 g·mol−1 | ||
| Appearance | Highly refractive colorless liquid | ||
| Odor | Sweet, minty, pleasant | ||
| Density | 1.564 g/cm3 (−20 °C) 1.489 g/cm3 (25 °C) 1.394 g/cm3 (60 °C) | ||
| Melting point | −63.5 °C (−82.3 °F; 209.7 K) | ||
| Boiling point | 61.15 °C (142.07 °F; 334.30 K) decomposes at 450 °C | ||
| 10.62 g/L (0 °C) 8.09 g/L (20 °C) 7.32 g/L (60 °C) | |||
| Solubility | Soluble in benzene Miscible in diethyl ether, oils, ligroin, alcohol, CCl4, CS2 | ||
| Solubility in acetone | ≥ 100 g/L (19 °C) | ||
| Solubility in dimethyl sulfoxide | ≥ 100 g/L (19 °C) | ||
| Vapor pressure | 0.62 kPa (−40 °C) 7.89 kPa (0 °C) 25.9 kPa (25 °C) 313 kPa (100 °C) 2.26 MPa (200 °C) | ||
Henry's law
constant (kH) |
3.67 L·atm/mol (24 °C) | ||
| Acidity (pKa) | 15.7 (20 °C) | ||
| UV-vis (λmax) | 250 nm, 260 nm, 280 nm | ||
| −59.30·10−6 cm3/mol | |||
| Thermal conductivity | 0.13 W/(m·K) (20 °C) | ||
Refractive index (nD)
|
1.4459 (20 °C) | ||
| Viscosity | 0.563 cP (20 °C) | ||
| Structure | |||
| Tetrahedral | |||
| 1.15 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
114.25 J/(mol·K) | ||
Std molar
entropy (S⦵298) |
202.9 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−134.3 kJ/mol | ||
Gibbs free energy (ΔfG⦵)
|
−71.1 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
473.21 kJ/mol | ||
| Pharmacology | |||
| N01AB02 (WHO) | |||
| Hazards[9] | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Decomposes to extremely toxic phosgene and hydrogen chloride in presence of light – possibly carcinogenic – Reproductive toxicity – hepatotoxic[3][4][5] | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H302, H315, H319, H331, H336, H351, H361d, H372 | |||
| P201, P202, P235, P260, P264, P270, P271, P280, P281, P301+P330+P331, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P311, P314, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Nonflammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
704 mg/kg (mouse, dermal)[6] | ||
LC50 (median concentration)
|
47,702 mg/m3 (rat, 4 hr)[7] | ||
LCLo (lowest published)
|
| ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
50 ppm (240 mg/m3)[4] | ||
REL (Recommended)
|
Ca ST 2 ppm (9.78 mg/m3) [60-minute][4] | ||
IDLH (Immediate danger)
|
500 ppm[4][clarification needed] | ||
| Safety data sheet (SDS) | [1] | ||
| Related compounds | |||
Related compounds
|
| ||
| Supplementary data page | |||
| Chloroform (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Chloroform,[10] or trichloromethane (often abbreviated as TCM), is an organochloride with the formula CHCl3 and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and PTFE.[11] Chloroform was once used as an inhalational anesthetic between the 19th century and the first half of the 20th century.[12][13] It is miscible with many solvents but it is only very slightly soluble in water (only 8 g/L at 20°C).
- ^ Gregory, William, A Handbook of Organic Chemistry (Third edition corrected and much extended), 1852, page 177
- ^ Daniel Pereira Gardner, Medicinal Chemistry for the Use of Students and the Profession: Being a Manual of the Science, with Its Applications to Toxicology, Physiology, Therapeutics, Hygiene, Etc (1848), page 271
- ^ "Part 3 Health Hazards" (PDF). Globally Harmonized System of Classification and Labelling of Chemicals (GHS). Second revised edition. United Nations. Archived (PDF) from the original on 4 March 2019. Retrieved 30 September 2017.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0127". National Institute for Occupational Safety and Health (NIOSH).
- ^ Toxicity on PubChem Archived 17 August 2018 at the Wayback Machine
- ^ Lewis, Richard J. (2012). Sax's Dangerous Properties of Industrial Materials (12th ed.). Wiley. ISBN 978-0-470-62325-1.
- ^ "Chloroform" (PDF). Environmental Protection Agency. September 2016. Retrieved 19 February 2024.
- ^ "Chloroform". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "PubChem: Safety and Hazards – GHS Classification". National Center for Biotechnology Information, U.S. National Library of Medicine. Archived from the original on 17 August 2018. Retrieved 17 August 2018.
- ^ "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 661. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The retained names 'bromoform' for HCBr3, 'chloroform' for HCCl3, and 'iodoform' for HCI3 are acceptable in general nomenclature. Preferred IUPAC names are substitutive names.
- ^ Rossberg, M.; et al. "Chlorinated Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_233.pub2. ISBN 978-3527306732.
- ^ "Ether and Chloroform". Archived from the original on 24 March 2018. Retrieved 24 April 2018.
- ^ "Chloroform [MAK Value Documentation, 2000]". The MAK-Collection for Occupational Health and Safety. 2012. pp. 20–58. doi:10.1002/3527600418.mb6766e0014. ISBN 978-3-527-60041-0.