| |
| Names | |
|---|---|
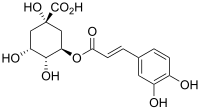
| Preferred IUPAC name
(1S,3R,4R,5R)-3-{[(2E)-3-(3,4-Dihydroxyphenyl)prop-2-enoyl]oxy}-1,4,5-trihydroxycyclohexane-1-carboxylic acid | |
| Other names
3-(3,4-Dihydroxycinnamoyl)quinate
3-(3,4-Dihydroxycinnamoyl)quinic acid 3-Caffeoylquinate 3-Caffeoylquinic acid 3-CQA 3-O-Caffeoylquinic acid Chlorogenate Chlorogenic acid Heriguard 3-trans-Caffeoylquinic acid 5-O-Caffeoylquinic acid | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H18O9 | |
| Molar mass | 354.311 g·mol−1 |
| Density | 1.28 g/cm3 |
| Melting point | 207 to 209 °C (405 to 408 °F; 480 to 482 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chlorogenic acid (CGA) is the ester of caffeic acid and (−)-quinic acid, functioning as an intermediate in lignin biosynthesis.[1] The term chlorogenic acids refers to a related polyphenol family of esters, including hydroxycinnamic acids (caffeic acid, ferulic acid and p-coumaric acid) with quinic acid.[2]
Despite the "chloro" of the name, chlorogenic acids contain no chlorine. Instead, the name comes from the Greek χλωρός (khloros, light green) and -γένος (genos, a suffix meaning "giving rise to"), pertaining to the green color produced when chlorogenic acids are oxidized.
- ^ Boerjan, Wout; Ralph, John; Baucher, Marie (2003). "Lignin biosynthesis". Annual Review of Plant Biology. 54: 519–546. doi:10.1146/annurev.arplant.54.031902.134938. PMID 14503002.
- ^ Clifford, M. N.; Johnston, K. L.; Knight, S.; Kuhnert, N. (2003). "Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids". Journal of Agricultural and Food Chemistry. 51 (10): 2900–2911. doi:10.1021/jf026187q. PMID 12720369.
