 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Largactil, Thorazine, Sonazine, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682040 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, rectal, intramuscular, intravenous |
| Drug class | Typical antipsychotic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 10–80% (Oral; large interindividual variation)[5] |
| Protein binding | 90–99%[5] |
| Metabolism | Liver, mostly CYP2D6-mediated[5] |
| Elimination half-life | 30 hours[6] |
| Excretion | Kidney (43–65% in 24 hrs)[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.042 |
| Chemical and physical data | |
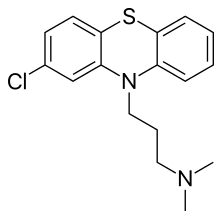
| Formula | C17H19ClN2S |
| Molar mass | 318.86 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Chlorpromazine (CPZ), marketed under the brand names Thorazine and Largactil among others, is an antipsychotic medication.[6] It is primarily used to treat psychotic disorders such as schizophrenia.[6] Other uses include the treatment of bipolar disorder, severe behavioral problems in children including those with attention deficit hyperactivity disorder, nausea and vomiting, anxiety before surgery, and hiccups that do not improve following other measures.[6] It can be given orally (by mouth), by intramuscular injection (injection into a muscle), or intravenously (injection into a vein).[6]
Chlorpromazine is in the typical antipsychotic class,[6] and, chemically, is one of the phenothiazines. Its mechanism of action is not entirely clear but is believed to be related to its ability as a dopamine antagonist.[6] It has antiserotonergic and antihistaminergic properties.[6]
Common side effects include movement problems, sleepiness, dry mouth, low blood pressure upon standing, and increased weight.[6] Serious side effects may include the potentially permanent movement disorder tardive dyskinesia, neuroleptic malignant syndrome, severe lowering of the seizure threshold, and low white blood cell levels.[6] In older people with psychosis as a result of dementia, it may increase the risk of death.[6] It is unclear if it is safe for use in pregnancy.[6]
Chlorpromazine was developed in 1950 and was the first antipsychotic on the market.[7][8] It is on the World Health Organization's List of Essential Medicines.[9][10] Its introduction has been labeled as one of the great advances in the history of psychiatry.[11][12] It is available as a generic medication.[6]
- ^ "Chlorpromazine Pregnancy and Breastfeeding Warnings". Drugs.com. 5 February 2020. Retrieved 21 August 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "List of nationally authorised medicinal products - Active substance: chlorpromazine: Procedure no.: PSUSA/00000715/202005" (PDF). Ema.europa.eu. Retrieved 3 March 2022.
- ^ a b c d "Australian Product Information – Largactil (chlorpromazine hydrochloride)" (PDF). Therapeutic Goods Administration (TGA). Sanofi Aventis Pty Ltd. 28 August 2012. Archived from the original on 30 March 2017. Retrieved 8 December 2013.
- ^ a b c d e f g h i j k l m "Chlorpromazine Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 1 December 2015.
- ^ López-Muñoz F, Alamo C, Cuenca E, Shen WW, Clervoy P, Rubio G (2005). "History of the discovery and clinical introduction of chlorpromazine". Annals of Clinical Psychiatry. 17 (3): 113–135. doi:10.1080/10401230591002002. PMID 16433053.
- ^ Ban TA (August 2007). "Fifty years chlorpromazine: a historical perspective". Neuropsychiatric Disease and Treatment. 3 (4): 495–500. PMC 2655089. PMID 19300578.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ López-Muñoz F, Alamo C, Cuenca E, Shen WW, Clervoy P, Rubio G (2005). "History of the discovery and clinical introduction of chlorpromazine". Annals of Clinical Psychiatry. 17 (3): 113–135. doi:10.1080/10401230591002002. PMID 16433053.
- ^ Shorter E (2005). A historical dictionary of psychiatry. New York: Oxford University Press. p. 6. ISBN 9780198039235. Archived from the original on 14 February 2017.