 | |
| Names | |
|---|---|
| Other names
chromium difluoride, chromium fluouride, chromous fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.140 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CrF2 | |
| Molar mass | 89.9929 g·mol−1 |
| Appearance | blue-green iridescent crystals[1] hygroscopic, turns to Cr2O3 when heated in air[1] |
| Density | 3.79 g/cm3[1] |
| Melting point | 894 °C (1,641 °F; 1,167 K)[1] |
| Boiling point | > 1,300 °C (2,370 °F; 1,570 K)[1] |
| 76.7 g/100 mL | |
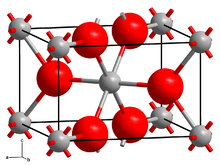
| Structure | |
| monoclinic[1] | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-8.645 kJ/g (solid) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chromium(II) fluoride is an inorganic compound with the formula CrF2. It exists as a blue-green iridescent solid. Chromium(II) fluoride is sparingly soluble in water, almost insoluble in alcohol, and is soluble in boiling hydrochloric acid, but is not attacked by hot distilled sulfuric acid or nitric acid. Like other chromous compounds, chromium(II) fluoride is oxidized to chromium(III) oxide in air.[2]
- ^ a b c d e f Perry, Dale L. (2011). Handbook of Inorganic Compounds, Second Edition. Boca Raton, Florida: CRC Press. p. 120. ISBN 978-1-43981462-8. Retrieved 2014-01-10.
- ^ Merck Index, 14 ed. entry 2245