| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Bis(η5-cyclopentadienyl)chromium(II)
| |||
| Other names
Dicyclopentadienylchromium(II)
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.013.670 | ||
| EC Number |
| ||
| 3366 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1325 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H10Cr | |||
| Molar mass | 182.186 g·mol−1 | ||
| Appearance | dark red crystals | ||
| Density | 1.43 g/cm3 | ||
| Melting point | 168 to 170 °C (334 to 338 °F; 441 to 443 K) | ||
| Boiling point | Sublimes (under vacuum) | ||
| Insoluble | |||
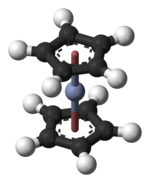
| Structure | |||
| Pseudooctahedral see Ferrocene | |||
| 0 D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Pyrophoric | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H302, H312, H314, H315, H317, H319, H332, H335 | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds
|
Fe(C5H5)2 Ni(C5H5)2 bis(benzene)chromium chromium(II) acetate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Chromocene is the organochromium compound with the formula [Cr(C5H5)2]. Like structurally related metallocenes, chromocene readily sublimes in a vacuum and is soluble in non-polar organic solvents. It is more formally known as bis(η5-cyclopentadienyl)chromium(II).[1]
- ^ Crabtree, R. H. (2009). The Organometallic Chemistry of the Transition Metals (5th ed.). Hoboken, NJ: John Wiley and Sons. p. 2. ISBN 978-0-470-25762-3.