 | |
| Clinical data | |
|---|---|
| Trade names | Nimbex |
| Other names | 51W89, cisatracurium besylate (USAN US) |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Metabolism | 80% Hofmann degradation/ liver |
| Elimination half-life | 20–29 minutes |
| Excretion | 10-15% unchanged |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.509 |
| Chemical and physical data | |
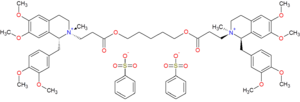
| Formula | C65H82N2O18S2 |
| Molar mass | 1243.49 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cisatracurium besilate (INN; cisatracurium besylate (USAN); formerly recognized as 51W89;[1] trade name Nimbex) is a bisbenzyltetrahydroisoquinolinium that has effect as a neuromuscular-blocking drug non-depolarizing neuromuscular-blocking drugs, used adjunctively in anesthesia to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation. It shows intermediate duration of action. Cisatracurium is one of the ten isomers of the parent molecule, atracurium.[2] Moreover, cisatracurium represents approximately 15% of the atracurium mixture.[3]
- ^ Meretoja OA, Taivainen T, Wirtavuori K (January 1995). "Pharmacodynamic effects of 51W89, an isomer of atracurium, in children during halothane anaesthesia". British Journal of Anaesthesia. 74 (1): 6–11. doi:10.1093/bja/74.1.6. PMID 7880708.
- ^ Stenlake JB, Waigh RD, Dewar GH, Dhar NC, Hughes R, Chapple DJ, Lindon JC, Ferrige AG (1984). "Biodegradable neuromuscular blocking agents. Part 6. Stereochemical studies on atracurium and related polyalkylene di-esters". Eur J Med Chem. 19 (5): 441–450.
- ^ Dear GJ, Harrelson JC, Jones AE, Johnson TE, Pleasance S (1995). "Identification of urinary and biliary conjugated metabolites of the neuromuscular blocker 51W89 by liquid chromatography/mass spectrometry". Rapid Communications in Mass Spectrometry. 9 (14): 1457–1464. Bibcode:1995RCMS....9.1457D. doi:10.1002/rcm.1290091425. PMID 8534894.