 | |
| Clinical data | |
|---|---|
| Trade names | Leustatin, Mavenclad, others[1] |
| Other names | 2-Chlorodeoxyadenosine; 2-Chloro-2'-deoxyadenosine; 2-CdA |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693015 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, subcutaneous (liquid), by mouth (tablet) |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (i.v.); 37 to 51% (orally)[5] |
| Protein binding | 25% (range 5-50%);[6] up to 20% (orally) [7] |
| Metabolism | Mostly via intracellular kinases; 15-18% is excreted unchanged.[6]
Intravenous and subcutaneous bolus injection: 15-18% is excreted unchanged After oral administration, 25% (±21%) of dose is excreted unchanged in urine and 3.8% as a metabolite.[7] |
| Elimination half-life | Approximately 10 hours after both intravenous infusion and subcutaneous bolus injection ranging from 5.6 to 7.6 hours[6] and 18.4 to 19.7 hours after oral administration, indicative of different elimination phases. |
| Excretion | Urinary[6] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.726 |
| Chemical and physical data | |
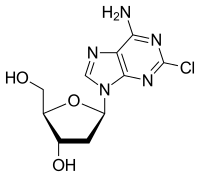
| Formula | C10H12ClN5O3 |
| Molar mass | 285.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cladribine, sold under the brand name Leustatin, among others, is a medication used to treat hairy cell leukemia (leukemic reticuloendotheliosis) and B-cell chronic lymphocytic leukemia.[8][9] Cladribine, sold under the brand name Mavenclad, is used for the treatment of adults with highly active forms of relapsing-remitting multiple sclerosis.[10]
Cladribine is a purine analogue that selectively targets and suppresses lymphocytes implicated in the underlying pathogenesis of multiple sclerosis and B-cell leukaemia.[11][7][12] Chemically, it mimics the nucleoside deoxyadenosine. However, unlike deoxyadenosine, it is relatively resistant to breakdown by the enzyme adenosine deaminase, which causes it to accumulate in targeted cells and interfere with the cell's ability to process DNA.[7] Cladribine is taken up by cells via transporter proteins. Once inside a cell, cladribine undergoes phosphorylation by the enzyme deoxycytidine kinase (DCK) to produce mononucleotide 2-chlorodeoxyadenosine 5’monophosphate (2-CdAMP), which is subsequently phosphorylated to the triphosphorylated active compound 2-chlorodeoxyadenosine 5’triphosphate (2-CdATP). Activated cladribine is incorporated into cellular DNA, which triggers apoptosis. Accumulation of cladribine into cells is dependent on the ratio of DCK and 5'-nucleotidase (5’-NT), which breaks down and inactivates the compound. This ratio differs between cell types, with high levels in T and B lymphocytes, resulting in selective targeting of these cells. In contrast, DCK:5'NT is relatively low in other cell types, thus sparing numerous non-haematological cells.[11][7]
It is on the World Health Organization's List of Essential Medicines.[13]
- ^ "Cladribine". Drugs.com. 28 February 2020. Retrieved 4 March 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Neurological therapies". Health Canada. 9 May 2018. Retrieved 13 April 2024.
- ^ "Mavenclad EPAR". European Medicines Agency (EMA). 22 August 2017. Retrieved 26 August 2024.
- ^ Liliemark J (February 1997). "The clinical pharmacokinetics of cladribine". Clinical Pharmacokinetics. 32 (2): 120–131. doi:10.2165/00003088-199732020-00003. PMID 9068927. S2CID 32926069.
- ^ a b c d "PRODUCT INFORMATION LITAK© 2 mg/mL solution for injection" (PDF). TGA eBusiness Services. St Leonards, Australia: Orphan Australia Pty. Ltd. 10 May 2010. Retrieved 27 November 2014.
- ^ a b c d e Giovannoni G (October 2017). "Cladribine to Treat Relapsing Forms of Multiple Sclerosis". Neurotherapeutics. 14 (4): 874–887. doi:10.1007/s13311-017-0573-4. PMC 5722776. PMID 29168160.
- ^ "European Medicines Agency - - Litak". www.ema.europa.eu. 17 September 2018. Archived from the original on 3 August 2018. Retrieved 1 July 2024.
- ^ "Leustat Injection. - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. Archived from the original on 3 October 2017. Retrieved 19 August 2016.
- ^ "Mavenclad EU SmPC" (PDF). European Medicines Agency. February 2021.
- ^ a b Leist TP, Weissert R (2011). "Cladribine: mode of action and implications for treatment of multiple sclerosis". Clinical Neuropharmacology. 34 (1): 28–35. doi:10.1097/WNF.0b013e318204cd90. PMID 21242742. S2CID 43201228.
- ^ Jain P, Pemmaraju N, Ravandi F (June 2014). "Update on the biology and treatment options for hairy cell leukemia". Current Treatment Options in Oncology. 15 (2): 187–209. doi:10.1007/s11864-014-0285-5. PMC 4198068. PMID 24652320.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.