A clathrate is a chemical substance consisting of a lattice that traps or contains molecules. The word clathrate is derived from the Latin clathratus (clatratus), meaning 'with bars, latticed'.[1] Most clathrate compounds are polymeric and completely envelop the guest molecule, but in modern usage clathrates also include host–guest complexes and inclusion compounds.[2] According to IUPAC, clathrates are inclusion compounds "in which the guest molecule is in a cage formed by the host molecule or by a lattice of host molecules."[3] The term refers to many molecular hosts, including calixarenes and cyclodextrins and even some inorganic polymers such as zeolites.
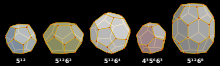
Clathrates can be divided into two categories: clathrate hydrates and inorganic clathrates. Each clathrate is made up of a framework and guests that reside the framework. Most common clathrate crystal structures can be composed of cavities such as dodecahedral, tetrakaidecahedral, and hexakaidecahedral cavities. Unlike hydrates, inorganic clathrates have a covalently bonded framework of inorganic atoms with guests typically consisting of alkali or alkaline earth metals. Due to the stronger covalent bonding, the cages are often smaller than hydrates. Guest atoms interact with the host by ionic or covalent bonds. Therefore, partial substitution of guest atoms follow Zintl rules so that the charge of the overall compound is conserved. Most inorganic clathrates have full occupancy of its framework cages by a guest atom to be in stable phase. Inorganic clathrates can be synthesized by direct reaction using ball milling at high temperatures or high pressures. Crystallization from melt is another common synthesis route. Due to the wide variety of composition of host and guest species, inorganic clathrates are much more chemically diverse and possess a wide range of properties. Most notably, inorganic clathrates can be found to be both an insulator and a superconductor (Ba8Si46). A common property of inorganic clathrates that has attracted researchers is low thermal conductivity. Low thermal conductivity is attributed to the ability of the guest atom to "rattle" within the host framework. The freedom of movement of the guest atoms scatters phonons that transport heat.[4]

- ^ Latin dictionary Archived 2012-04-14 at the Wayback Machine
- ^ Atwood, J. L. (2012) "Inclusion Compounds" in Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim. doi:10.1002/14356007.a14_119
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "clathrates". doi:10.1351/goldbook.C01097
- ^ a b c Krishna, Lakshmi; Koh, Carolyn A. (February 2015). "Inorganic and methane clathrates: Versatility of guest–host compounds for energy harvesting". MRS Energy & Sustainability. 2 (1): 8. doi:10.1557/mre.2015.9. ISSN 2329-2229.