 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /klɪndəˈmaɪsɪn/ |
| Trade names | Cleocin, Clinacin, Dalacin, others |
| Other names | 7-chloro-lincomycin 7-chloro-7-deoxylincomycin, DARE-BV1 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682399 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, topical, intravenous, intravaginal |
| Drug class | Lincosamide antibiotic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90% (by mouth) 4–5% (topical) |
| Protein binding | 95% |
| Metabolism | Liver |
| Elimination half-life | 2–3 hour |
| Excretion | Bile duct and kidney (around 20%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.038.357 |
| Chemical and physical data | |
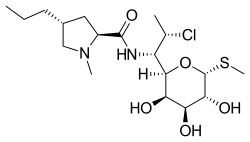
| Formula | C18H33ClN2O5S |
| Molar mass | 424.98 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Clindamycin is a lincosamide antibiotic medication used for the treatment of a number of bacterial infections, including osteomyelitis (bone) or joint infections, pelvic inflammatory disease, strep throat, pneumonia, acute otitis media (middle ear infections), and endocarditis.[5] It can also be used to treat acne,[5][6] and some cases of methicillin-resistant Staphylococcus aureus (MRSA).[7] In combination with quinine, it can be used to treat malaria.[5][6] It is available by mouth, by injection into a vein, and as a cream or a gel to be applied to the skin or in the vagina.[4][5][6][8][9]
Common side effects include nausea and vomiting, diarrhea, skin rashes, and pain at the site of injection.[5] It increases the risk of hospital-acquired Clostridioides difficile colitis about fourfold and thus is only recommended for use when other antibiotics are not appropriate.[10][5] It appears to be generally safe in pregnancy.[5] It is of the lincosamide class and works by blocking bacteria from making protein.[5]
Clindamycin was first made in 1966 from lincomycin.[11][12] It is on the World Health Organization's List of Essential Medicines.[13] It is available as a generic medication.[14][15] In 2022, it was the 147th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[16][17]
- ^ Use During Pregnancy and Breastfeeding
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Product monograph brand safety updates". Health Canada. 6 June 2024. Retrieved 8 June 2024.
- ^ a b "Xaciato- clindamycin phosphate gel". DailyMed. Retrieved 24 December 2021.
- ^ a b c d e f g h "Clindamycin (Systemic)". The American Society of Health-System Pharmacists. Archived from the original on 12 August 2021. Retrieved 19 December 2021.
- ^ a b c Leyden JJ (2006). Hidradenitis suppurativa. Berlin: Springer. p. 152. ISBN 9783540331018. Archived from the original on 8 September 2017.
- ^ Daum RS (July 2007). "Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus". N. Engl. J. Med. 357 (4): 380–90. doi:10.1056/NEJMcp070747. PMID 17652653.
- ^ "Clindamycin phosphate- clindamycin phosphate gel usp, 1% gel". DailyMed. Retrieved 19 December 2021.
- ^ "Daré Announces FDA Approval of Xaciato (clindamycin phosphate) Vaginal Gel as a Treatment for Bacterial Vaginosis". Daré Bioscience (Press release). 7 December 2021. Retrieved 19 December 2021.
- ^ Thomas C, Stevenson M, Riley TV (2003). "Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review". J Antimicrob Chemother. 51 (6): 1339–50. doi:10.1093/jac/dkg254. PMID 12746372.
- ^ Smieja M (January 1998). "Current indications for the use of clindamycin: A critical review". The Canadian Journal of Infectious Diseases. 9 (1): 22–8. doi:10.1155/1998/538090. PMC 3250868. PMID 22346533.
- ^ Neonatal Formulary: Drug Use in Pregnancy and the First Year of Life (7 ed.). John Wiley & Sons. 2014. p. 162. ISBN 9781118819517. Archived from the original on 8 September 2017.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 108. ISBN 9781284057560.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Clindamycin Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.