 | |
| Clinical data | |
|---|---|
| Trade names | Clolar, Evoltra |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607012 |
| License data |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.159.663 |
| Chemical and physical data | |
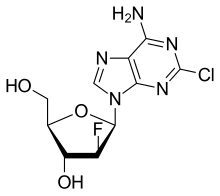
| Formula | C10H11ClFN5O3 |
| Molar mass | 303.68 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Clofarabine is a purine nucleoside antimetabolite marketed in the United States and Canada as Clolar. In Europe and Australia/New Zealand the product is marketed under the name Evoltra. It is FDA-approved for treating relapsed or refractory acute lymphoblastic leukaemia (ALL) in children after at least two other types of treatment have failed. Some investigations of effectiveness in cases of acute myeloid leukaemia (AML) and juvenile myelomonocytic leukaemia (JMML) have been carried out. Ongoing trials are assessing its efficacy for managing other cancers.
- ^ "Clolar- clofarabine injection". DailyMed. 31 December 2019. Retrieved 27 September 2020.
- ^ "Evoltra EPAR". European Medicines Agency (EMA). Retrieved 27 September 2020.