| |

| |
| Names | |
|---|---|
| IUPAC name
Cobalt(II) acetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.687 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
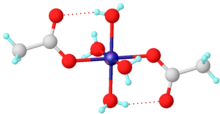
| Co(C2H3O2)2 | |
| Molar mass | 177.02124 g/mol (anhydrous) 249.08 g/mol (tetrahydrate) |
| Appearance | Pink crystals (anhydrous) intense red crystals (tetrahydrate) |
| Odor | vinegar (tetrahydrate) |
| Density | 1.705 g/cm3 (tetrahydrate) |
| Melting point | 140 °C (284 °F; 413 K) (tetrahydrate) |
| Soluble | |
| Solubility | soluble in alcohol, dilute acids, pentyl acetate (tetrahydrate) |
| +11,000·10−6 cm3/mol | |
Refractive index (nD)
|
1.542 (tetrahydrate) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
503 mg/kg (oral, rat) |
| Safety data sheet (SDS) | J.T. Baker MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cobalt(II) acetate is the cobalt salt of acetic acid. It is commonly found as the tetrahydrate Co(CH3CO2)2·4 H2O, abbreviated Co(OAc)2·4 H2O. It is used as a catalyst.
