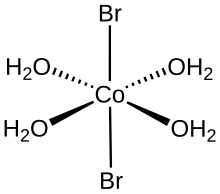
 Structure of cobalt(II) bromide tetrahydrate
| |
 Crystal structure of cobalt(II) bromide
| |
 Anhydrous cobalt(II) bromide in a vial
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.242 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CoBr2, CoBr2.6H2O, CoBr2.2H2O | |
| Molar mass | 218.7412 g/mol (anhydrous) 326.74 g/mol (hexahydrate) |
| Appearance | Bright green crystals (anhydrous) Red-purple crystals (hexahydrate) |
| Density | 4.909 g/cm3 (anhydrous) 2.46 g/cm3 (hexahydrate) |
| Melting point | 678 °C (1,252 °F; 951 K) (anhydrous) 47 °C (hexahydrate) |
| anhydrous: 66.7 g/100 mL (59 °C) 68.1 g/100 mL (97 °C) hexahydrate: 113.2 g/100 mL (20 °C) | |
| Solubility | 77.1 g/100 mL (ethanol, 20 °C) 58.6 g/100 mL (methanol, 30 °C) soluble in methyl acetate, ether, alcohol, acetone |
| +13000·10−6 cm3/mol | |
| Structure | |
| Rhombohedral, hP3, SpaceGroup = P-3m1, No. 164 | |
| octahedral | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H302, H312, H315, H317, H319, H332, H334, H335, H350 | |
| P201, P202, P261, P264, P270, P271, P272, P280, P281, P285, P301+P312, P302+P352, P304+P312, P304+P340, P304+P341, P305+P351+P338, P308+P313, P312, P321, P322, P330, P332+P313, P333+P313, P337+P313, P342+P311, P362, P363, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
406 mg/kg (oral, rat) |
| Safety data sheet (SDS) | Fisher Scientific |
| Related compounds | |
Other anions
|
cobalt(II) fluoride cobalt(II) chloride cobalt(II) iodide |
Other cations
|
iron(II) bromide nickel(II) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cobalt(II) bromide (CoBr2) is an inorganic compound. In its anhydrous form, it is a green solid that is soluble in water, used primarily as a catalyst in some processes.
