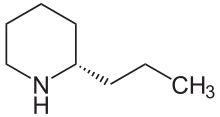
 (S)-Coniine
| |
| |
| Names | |
|---|---|
| IUPAC name
(2S)-2-Propylpiperidine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.006.621 |
| EC Number |
|
| KEGG |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H17N | |
| Molar mass | 127.231 g·mol−1 |
| Appearance | Colorless, oily liquid |
| Melting point | −2 °C (28 °F; 271 K) |
| Boiling point | 166 to 167 °C (331 to 333 °F; 439 to 440 K) |
Refractive index (nD)
|
1.4505 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Coniine is a poisonous chemical compound, an alkaloid present in and isolable from poison hemlock (Conium maculatum), where its presence has been a source of significant economic, medical, and historico-cultural interest; coniine is also produced by the yellow pitcher plant (Sarracenia flava), and fool's parsley (Aethusa cynapium). Its ingestion and extended exposure are toxic to humans and all classes of livestock; its mechanism of poisoning involves disruption of the central nervous system, with death caused by respiratory paralysis. The biosynthesis of coniine contains as its penultimate step the non-enzymatic cyclisation of 5-oxooctylamine to γ-coniceine, a Schiff base differing from coniine only by its carbon-nitrogen double bond in the ring. This pathway results in natural coniine that is a mixture—a racemate—composed of two enantiomers, the stereoisomers (S)-(+)-coniine and (R)-(−)-coniine, depending on the direction taken by the chain that branches from the ring. Both enantiomers are toxic, with the (R)-enantiomer being the more biologically active and toxic of the two in general. Coniine holds a place in organic chemistry history as being the first of the important class of alkaloids to be synthesized, by Albert Ladenburg in 1886, and it has been synthesized in the laboratory in a number of unique ways through to modern times.
Hemlock poisoning has been a periodic human concern, a regular veterinary concern, and has had significant occurrences in human and cultural history. Notably, in 399 BC, Socrates was sentenced to death by drinking a coniine-containing mixture of poison hemlock.