| NuvaRing | |
|---|---|
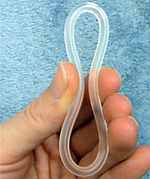 NuvaRing is a flexible plastic ring with both oestrogen and progesterone. | |
| Background | |
| Type | Hormonal |
| First use | 2001 |
| Failure rates (first year) | |
| Perfect use | 0.3 for Nuvaring%[1] |
| Typical use | 1.5 to 9 for Nuvaring%[2][1] |
| Usage | |
| Duration effect | 4 weeks for Nuvaring, 3 months for progesterone only vaginal ring |
| User reminders | Inserted for 3 weeks and then removed for 7 days for Nuvaring |
| Clinic review | Annual |
| Advantages and disadvantages | |
| STI protection | No |
| Weight | No proven effect |
| Benefits | Easy insertion and removal |
A contraceptive vaginal ring is a type of hormonal insert that is placed in the vagina for the purpose of birth control. The rings themselves utilize a plastic polymer matrix that is inlaid or embedded with contraceptive drug.[3] This drug, often one or two hormones, is absorbed directly through the bloodstream through the cells that line the vaginal wall.[4][5] Some vaginal rings contain both an estrogen and a progestin (brand names NuvaRing and Annovera), which are available in Europe and the United States.[6][7][8][9] Other vaginal rings contain just progesterone (brand name Progering).[10] The progesterone-only ring is only available in Latin America, exclusively for postpartum breastfeeding parents.[9]
The Progering is made of silicone-elastorone with an outer diameter of 58 mm and cross-sectional diameter of 8.4 mm.[10] Similarly, Annovera has an outer diameter of 56 mm and cross-sectional diameter of 8.4 mm.[7] In contrast, the Nuvaring has a diameter of 54 mm with a cross-sectional diameter that measures 4 mm.[6] The vaginal rings work as a long acting drug delivery system for varying indications, including prevention of pregnancy, improvement of dysmenorrhea and menorrhagia, lower risks of ovarian and endometrial cancers, and reduction of risk of cysts in the ovaries.[11] Although the vaginal rings do not provide protection for sexually transmitted diseases, the rings are being assessed as a possible drug delivery system for HIV prevention.[12]
- ^ a b Trussell J (2011). "Contraceptive efficacy". In Hatcher RA, Trussell J, Nelson AL, Cates Jr W, Kowal D, Policar MS (eds.). Contraceptive technology (20th revised ed.). New York: Ardent Media. pp. 779–863. ISBN 978-1-59708-004-0. ISSN 0091-9721. OCLC 781956734. "Contraception Failure Table" (PDF). Archived from the original (PDF) on 2017-02-15.
Table 3–2 Percentage of women experiencing an unintended pregnancy during the first year of typical use and the first year of perfect use of contraception, and the percentage continuing use at the end of the first year. United States.
- ^ Cite error: The named reference
Whit2014was invoked but never defined (see the help page). - ^ Kar A, Ahamad N, Dewani M, Awasthi L, Patil R, Banerjee R (April 2022). "Wearable and implantable devices for drug delivery: Applications and challenges". Biomaterials. 283: 121435. doi:10.1016/j.biomaterials.2022.121435. PMID 35227964. S2CID 247100171.
- ^ Krovi SA, Johnson LM, Luecke E, Achilles SL, van der Straten A (September 2021). "Advances in long-acting injectables, implants, and vaginal rings for contraception and HIV prevention". Advanced Drug Delivery Reviews. 176: 113849. doi:10.1016/j.addr.2021.113849. PMID 34186143. S2CID 235685856.
- ^ Cite error: The named reference
:7was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
:0was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
:1was invoked but never defined (see the help page). - ^ "Vaginal ring". nhs.uk. December 2017. Archived from the original on 25 August 2019. Retrieved 25 August 2019.
- ^ a b "Do Women Find the Progesterone Vaginal Ring Acceptable? Findings from Kenya, Nigeria, and Senegal". fp2030.org. 2019-02-11. Retrieved 2022-08-04.
- ^ a b Carr SL, Gaffield ME, Dragoman MV, Phillips S (September 2016). "Safety of the progesterone-releasing vaginal ring (PVR) among lactating women: A systematic review". Contraception. 94 (3): 253–261. doi:10.1016/j.contraception.2015.04.001. PMID 25869631.
- ^ Cite error: The named reference
:8was invoked but never defined (see the help page). - ^ Ridgeway K, Montgomery ET, Smith K, Torjesen K, van der Straten A, Achilles SL, Griffin JB (February 2022). "Vaginal ring acceptability: A systematic review and meta-analysis of vaginal ring experiences from around the world". Contraception. 106: 16–33. doi:10.1016/j.contraception.2021.10.001. PMC 9128798. PMID 34644609. Archived from the original on 2022-08-03. Retrieved 2022-08-01.