| Cope rearrangement | |
|---|---|
| Named after | Arthur C. Cope |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| Organic Chemistry Portal | cope-rearrangement |
| RSC ontology ID | RXNO:0000028 |
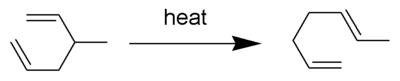
The Cope rearrangement is an extensively studied organic reaction involving the [3,3]-sigmatropic rearrangement of 1,5-dienes.[1][2][3][4] It was developed by Arthur C. Cope and Elizabeth Hardy. For example, 3-methyl-hexa-1,5-diene heated to 300 °C yields hepta-1,5-diene.
The Cope rearrangement causes the fluxional states of the molecules in the bullvalene family.
- ^ Arthur C. Cope; Elizabeth M. Hardy; J. Am. Chem. Soc. 1940, 62, 441.
- ^ Rhoads, S. J.; Raulins, N. R.; Org. React. 1975, 22, 1–252. (Review)
- ^ Hill, R. K.; Compr. Org. Synth. 1991, 5, 785–826.
- ^ Wilson, S. R.; Org. React. 1993, 43, 93–250. (Review)