| |
| Names | |
|---|---|
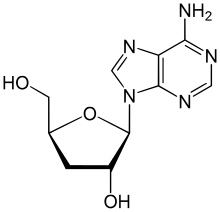
| IUPAC name
3′-Deoxyadenosine
| |
| Systematic IUPAC name
(2S,3R,5S)-2-(6-Amino-9H-purin-9-yl)-5-(hydroxymethyl)oxolan-3-ol | |
| Other names
Cordycepine
9-(3-Deoxy-β-D-ribofuranosyl)adenine 3-dA | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.720 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H13N5O3 | |
| Molar mass | 251.246 g·mol−1 |
| Melting point | 225.5 °C (437.9 °F; 498.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cordycepin, or 3'-deoxyadenosine, is a derivative of the nucleoside adenosine, differing from the latter by the replacement of the hydroxy group in the 3' position with a hydrogen. It was initially extracted from the fungus Cordyceps militaris,[1] but can now be produced synthetically.[2] It is also found in other Cordyceps species as well as Ophiocordyceps sinensis.[3]
- ^ Cunningham, K. G., Manson, W., Spring, F. S., Hutchinson, S. A. (1950). "Cordycepin, a Metabolic Product isolated from Cultures of Cordyceps militaris (Linn.) Link". Nature. 166 (4231): 949. Bibcode:1950Natur.166..949C. doi:10.1038/166949a0. PMID 14796634.
- ^ Huang S, Liu H, Sun Y, Chen J, Li X, Xu J, Hu Y, Li Y, Deng Z, Zhong S (2018-01-01). "An effective and convenient synthesis of cordycepin from adenosine". Chemical Papers. 72 (1): 149–160. doi:10.1007/s11696-017-0266-9. ISSN 1336-9075. S2CID 90915876.
- ^ Zhou X, Luo L, Dressel W, Shadier G, Krumbiegel D, Schmidtke P, Zepp F, Meyer CU (2008). "Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis". The American Journal of Chinese Medicine. 36 (5): 967–80. doi:10.1142/S0192415X08006387. PMID 19051361.