| |
| |
| Names | |
|---|---|
| Other names
Pyridinium dichromate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.039.511 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H12N2Cr2O7 | |
| Molar mass | 376.2 g/mol |
| Appearance | orange to brown solid[1] |
| Boiling point | 145 to 147 °C (293 to 297 °F; 418 to 420 K)[1] |
| soluble in water[1] | |
| Hazards | |
| GHS labelling: | |
     
| |
| Danger | |
| H228, H272, H314, H315, H317, H319, H350, H410 | |
| P201, P202, P210, P220, P221, P240, P241, P260, P261, P264, P272, P273, P280, P281, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P321, P332+P313, P333+P313, P337+P313, P362, P363, P370+P378, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
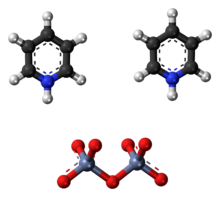
The Cornforth reagent (pyridinium dichromate or PDC) is a pyridinium salt of dichromate with the chemical formula [C5H5NH]2[Cr2O7]. This compound is named after the Australian-British chemist Sir John Warcup Cornforth (b. 1917) who introduced it in 1962.[2][3] The Cornforth reagent is a strong oxidizing agent which can convert primary and secondary alcohols to aldehydes and ketones respectively. In its chemical structure and functions it is closely related to other compounds made from hexavalent chromium oxide, such as pyridinium chlorochromate and Collins reagent. Because of their toxicity, these reagents are rarely used nowadays.[4]
- ^ a b c Pyridinium dichromate, MSDS, Alfa Caesar
- ^ Alexander Senning Elsevier's dictionary of chemoetymology: the whies and whences of chemical nomenclature and terminology, Elsevier, 2007, ISBN 0-444-52239-5 p. 94
- ^ Cornforth, R.H.; Cornforth, J.W.; Popjak, G. (1962). "Preparation of R-and S-mevalonolactones". Tetrahedron. 18 (12): 1351–4. doi:10.1016/S0040-4020(01)99289-0.
- ^ G. Tojo; M. Fernâandez (2006). Oxidation of alcohols to aldehydes and ketones: a guide to current common practice. New York: Springer. pp. 28, 29, 86. ISBN 0-387-23607-4.