Cosegregation is the transmission to the next generation, of two or more genes in proximity on the same chromosome. Their closeness means that they are genetically linked.[1] It may also represent an interaction estimation probability between any number of loci.
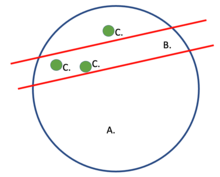
Interaction probability is determined using specified parts of a target gene (loci) and a group of nuclear profiles (NPs).[2] The picture to the right serves to provide visual aid as to how a slice (NP) is taken from the nucleus and loci are searched for within the NP. Cosegregation used within other mathematical models (SLICE[3] and normalized linkage disequilibrium) assist in rendering 3-D visualizations as a smaller process of genome architecture mapping (GAM). These renderings help determine genomic density and radial position.
|
- ^ "Cosegregation". cancer.gov. Retrieved 4 May 2023.
- ^ Wrighton, Katharine H. (May 2017). "Zooming in on nuclear organization". Nature Reviews Molecular Cell Biology. 18 (5): 275. doi:10.1038/nrm.2017.28. PMID 28327555. S2CID 3453730.
- ^ a b Beagrie, Robert A.; Scialdone, Antonio; Schueler, Markus; Kraemer, Dorothee C. A.; Chotalia, Mita; Xie, Sheila Q.; Barbieri, Mariano; de Santiago, Inês; Lavitas, Liron-Mark; Branco, Miguel R.; Fraser, James; Dostie, Josée; Game, Laurence; Dillon, Niall; Edwards, Paul A. W.; Nicodemi, Mario; Pombo, Ana (March 2017). "Complex multi-enhancer contacts captured by genome architecture mapping". Nature. 543 (7646): 519–524. Bibcode:2017Natur.543..519B. doi:10.1038/nature21411. PMC 5366070. PMID 28273065.
- ^ Mohammadi, Leila; Vreeswijk, Maaike P; Oldenburg, Rogier; van den Ouweland, Ans; Oosterwijk, Jan C; van der Hout, Annemarie H; Hoogerbrugge, Nicoline; Ligtenberg, Marjolijn; Ausems, Margreet G; van der Luijt, Rob B; Dommering, Charlotte J; Gille, Johan J; Verhoef, Senno; Hogervorst, Frans B; van Os, Theo A; Gómez García, Encarna; Blok, Marinus J; Wijnen, Juul T; Helmer, Quinta; Devilee, Peter; van Asperen, Christi J; van Houwelingen, Hans C (29 June 2009). "A simple method for co-segregation analysis to evaluate the pathogenicity of unclassified variants; BRCA1 and BRCA2 as an example". BMC Cancer. 9: 211. doi:10.1186/1471-2407-9-211. PMC 2714556. PMID 19563646.
- ^ Belman, Sophie; Parsons, Michael T.; Spurdle, Amanda B.; Goldgar, David E.; Feng, Bing-Jian (December 2020). "Considerations in assessing germline variant pathogenicity using cosegregation analysis". Genetics in Medicine. 22 (12): 2052–2059. doi:10.1038/s41436-020-0920-4. PMID 32773770. S2CID 221084291.