| |
| Names | |
|---|---|
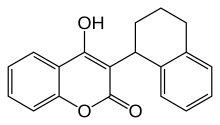
| IUPAC name
(RS)-2-Hydroxy-3-(1,2,3,4-tetrahydronaphthalen-1-yl)-4H-chromen-4-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.024.931 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H16O3 | |
| Molar mass | 292.334 g·mol−1 |
| Hazards | |
| GHS labelling: | |
   [1] [1]
| |
| Danger[1] | |
| H300, H311, H330, H360D, H372, H410[1] | |
| P201, P202, P260, P264, P270, P271, P273, P280, P281, P284, P301+P310, P302+P352, P304+P340, P308+P313, P310, P312, P314, P320, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Coumatetralyl is an anticoagulant of the 4-hydroxycoumarin vitamin K antagonist type used as a rodenticide.[2]
- ^ a b c d "Coumatetralyl". PubChem. National Center for Biotechnology Information. Retrieved 2021-11-25.
- ^ J. Routt Reigart and James R. Roberts, ed. (2013). "Chapter 18: Rodenticides". Recognition and Management of Pesticide Poisonings (PDF) (6th ed.).