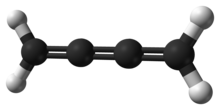
A cumulene is a compound having three or more cumulative (consecutive) double bonds.[1] They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (H2C=C=C=CH2), which is also called simply cumulene. Unlike most alkanes and alkenes, cumulenes tend to be rigid, comparable to polyynes. Cumulene carbenes H2Cn for n from 3 to 6 have been observed in interstellar molecular clouds[2][3] and in laboratory experiments[4] by using microwave and infrared spectroscopy. (The more stable cumulenes H2CnH2 are difficult to detect optically because they lack an electric dipole moment.) Cumulenes containing heteroatoms are called heterocumulenes;[5] an example is carbon suboxide.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "cumulenes". doi:10.1351/goldbook.C01440
- ^ Thaddeus, P.; Gottlieb, C.A.; Mollaaghababa, R.; Vrtilek, J.M. (1993). "Free carbenes in the interstellar gas". J. Chem. Soc., Faraday Trans. 89 (13): 2125. doi:10.1039/ft9938902125. ISSN 0956-5000.
- ^ Cabezas, C.; Tercero, B.; Agúndez, M.; et al. (2021). "Cumulene carbenes in TMC-1: Astronomical discovery of I\-H2C5". Astronomy & Astrophysics. 650: L9. doi:10.1051/0004-6361/202141274. ISSN 0004-6361. PMC 7611420.
- ^ McCarthy, M. C.; Travers, M. J.; Kovács, A.; et al. (1997). "Detection and Characterization of the Cumulene Carbenes H2C5 and H2C6". Science. 275 (5299): 518–520. doi:10.1126/science.275.5299.518. ISSN 0036-8075.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "heterocumulenes". doi:10.1351/goldbook.H02797