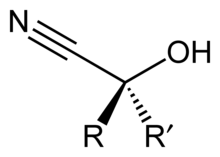
In organic chemistry, a cyanohydrin or hydroxynitrile is a functional group found in organic compounds in which a cyano and a hydroxy group are attached to the same carbon atom. The general formula is R2C(OH)CN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids. Cyanohydrins can be formed by the cyanohydrin reaction, which involves treating a ketone or an aldehyde with hydrogen cyanide (HCN) in the presence of excess amounts of sodium cyanide (NaCN) as a catalyst:[1]
- RR’C=O + HCN → RR’C(OH)CN
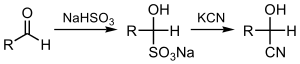
In this reaction, the nucleophilic CN− ion attacks the electrophilic carbonyl carbon in the ketone, followed by protonation by HCN, thereby regenerating the cyanide anion. Cyanohydrins are also prepared by displacement of sulfite by cyanide salts:[2]
Cyanohydrins are intermediates in the Strecker amino acid synthesis. In aqueous acid, they are hydrolyzed to the α-hydroxy acid.
- ^ David T. Mowry (1948). "The Preparation of Nitriles". Chem. Rev. 42 (2): 189–283. doi:10.1021/cr60132a001. PMID 18914000.
- ^ Corson, B. B.; Dodge, R. A.; Harris, S. A.; Yeaw, J. S. (1941). "Mandelic Acid". Organic Syntheses; Collected Volumes, vol. 1, p. 336.