 | |
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
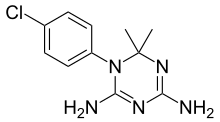
| Formula | C11H14ClN5 |
| Molar mass | 251.72 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cycloguanil is a dihydrofolate reductase inhibitor,[1] and is a metabolite of the antimalarial drug proguanil; its formation in vivo has been thought to be primarily responsible for the antimalarial activity of proguanil.[2] However, more recent work has indicated that, while proguanil is synergistic with the drug atovaquone (as in the combination Malarone), cycloguanil is in fact antagonistic to the effects of atovaquone, suggesting that, unlike cycloguanil, proguanil may have an alternative mechanism of antimalarial action besides dihydrofolate reductase inhibition.[3]
Although cycloguanil is not currently in general use as an antimalarial, the continuing development of resistance to current antimalarial drugs has led to renewed interest in studying the use of cycloguanil in combination with other drugs.[4]
- ^ Srivastava IK, Vaidya AB (June 1999). "A mechanism for the synergistic antimalarial action of atovaquone and proguanil". Antimicrobial Agents and Chemotherapy. 43 (6): 1334–9. doi:10.1128/AAC.43.6.1334. PMC 89274. PMID 10348748.
- ^ Watkins WM, Sixsmith DG, Chulay JD (June 1984). "The activity of proguanil and its metabolites, cycloguanil and p-chlorophenylbiguanide, against Plasmodium falciparum in vitro" (Free full text). Annals of Tropical Medicine and Parasitology. 78 (3): 273–8. doi:10.1080/00034983.1984.11811816. PMID 6385887.
- ^ Thapar MM, Gupta S, Spindler C, Wernsdorfer WH, Björkman A (May 2003). "Pharmacodynamic interactions among atovaquone, proguanil and cycloguanil against Plasmodium falciparum in vitro". Transactions of the Royal Society of Tropical Medicine and Hygiene. 97 (3): 331–7. doi:10.1016/S0035-9203(03)90162-3. PMID 15228254.
- ^ Walzer PD, Foy J, Steele P, White M (July 1993). "Synergistic combinations of Ro 11-8958 and other dihydrofolate reductase inhibitors with sulfamethoxazole and dapsone for therapy of experimental pneumocystosis". Antimicrobial Agents and Chemotherapy. 37 (7): 1436–43. doi:10.1128/AAC.37.7.1436. PMC 187990. PMID 8363372.