| |

| |
| Names | |
|---|---|
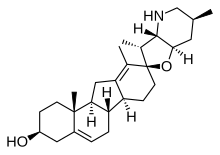
| IUPAC name
17,23β-Epoxyveratraman-3β-ol
| |
| Systematic IUPAC name
(2′R,3S,3′R,3′aS,6′S,6aS,6bS,7′aR,11aS,11bR)-3′,6′,10,11b-Tetramethyl-1,2,3,3′a,4,4′,5′,6,6′,6a,6b,7,7′,7′a,8,11,11a,11b-octadecahydro-3′H-spiro[benzo[a]fluorene-9,2′-furo[3,2-b]pyridin]-3-ol | |
| Other names
• 11-Deoxojervine
• (3β,23R)-17,23-Epoxyveratraman-3-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.156.363 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C27H41NO2 | |
| Molar mass | 411.630 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cyclopamine (11-deoxojervine) is a naturally occurring steroidal alkaloid. It is a teratogenic component of corn lily (Veratrum californicum), which when consumed during gestation has been demonstrated to induce birth defects, including the development of a single eye (cyclopia) in offspring.[1] The molecule was named after this effect, which was originally observed by Idaho lamb farmers in 1957 after their herds gave birth to cycloptic lambs. It then took more than a decade to identify corn lily as the culprit.[2] Later work suggested that differing rain patterns had changed grazing behaviours, which led to a greater quantity of corn lily to be ingested by pregnant sheep.[3] Cyclopamine interrupts the sonic hedgehog signalling pathway, instrumental in early development, ultimately causing birth defects.
- ^ Chen, James K. (2016). "I only have eye for ewe: the discovery of cyclopamine and development of Hedgehog pathway-targeting drugs". Natural Product Reports. 33 (5): 595–601. doi:10.1039/C5NP00153F. ISSN 0265-0568. PMC 4856577. PMID 26787175.
- ^ "The strange case of the cyclops sheep - Tien Nguyen". TED-Ed. Retrieved 2018-04-27.
- ^ Heretsch P, Tzagkaroulaki L, Giannis A (May 2010). "Cyclopamine and hedgehog signaling: chemistry, biology, medical perspectives". Angewandte Chemie. 49 (20): 3418–27. doi:10.1002/anie.200906967. PMID 20429080.