
| |
| |

| |
| Names | |
|---|---|
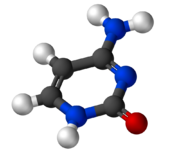
| Preferred IUPAC name
4-Aminopyrimidin-2(1H)-one | |
| Other names
4-Amino-1H-pyrimidine-2-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.681 |
| KEGG | |
| MeSH | Cytosine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H5N3O | |
| Molar mass | 111.10 g/mol |
| Density | 1.55 g/cm3 (calculated) |
| Melting point | 320 to 325 °C (608 to 617 °F; 593 to 598 K) (decomposes) |
| Acidity (pKa) | 4.45 (secondary), 12.2 (primary)[1] |
| -55.8·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cytosine (/ˈsaɪtəˌsiːn, -ˌziːn, -ˌsɪn/[2][3]) (symbol C or Cyt) is one of the four nucleotide bases found in DNA and RNA, along with adenine, guanine, and thymine (uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached (an amine group at position 4 and a keto group at position 2). The nucleoside of cytosine is cytidine. In Watson–Crick base pairing, it forms three hydrogen bonds with guanine.
- ^ Dawson, R.M.C.; et al. (1959). Data for Biochemical Research. Oxford: Clarendon Press.
- ^ "Cytosine". Dictionary.com Unabridged (Online). n.d.
- ^ "Cytosine". Merriam-Webster.com Dictionary. Merriam-Webster.