This article contains too many or overly lengthy quotations. (May 2022) |
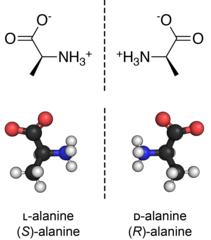
D-Amino acids are amino acids where the stereogenic carbon alpha to the amino group has the D-configuration. For most naturally-occurring amino acids, this carbon has the L-configuration. D-Amino acids are occasionally found in nature as residues in proteins. They are formed from ribosomally-derived D-amino acid residues.[1]
Amino acids, as components of peptides, peptide hormones, structural and immune proteins, are the most important bioregulators involved in all life processes along with nucleic acids, carbohydrates and lipids. "Environmental ᴅ-amino acids are thought to be derived from organic diagenesis such as racemization and release from bacterial cell walls and even from microbial production."[2]
- ^ Genchi G (September 2017). "An overview on D-amino acids". Amino Acids. 49 (9): 1521–1533. doi:10.1007/s00726-017-2459-5. PMID 28681245. S2CID 3998765.
- ^ Naganuma, Takeshi; Iinuma, Yoshiakira; Nishiwaki, Hitomi; Murase, Ryota; Masaki, Kazuo; Nakai, Ryosuke (2018). "Enhanced Bacterial Growth and Gene Expression of D-Amino Acid Dehydrogenase With D-Glutamate as the Sole Carbon Source". Frontiers in Microbiology. 9: 2097. doi:10.3389/fmicb.2018.02097. ISSN 1664-302X. PMC 6131576. PMID 30233558.
