 | |
| Clinical data | |
|---|---|
| Pronunciation | /dəˈklætəsvɪər/ də-KLAT-əs-veer |
| Trade names | Daklinza |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a615044 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 67%[4] |
| Protein binding | 99%[4] |
| Metabolism | CYP3A |
| Elimination half-life | 12–15 hours |
| Excretion | Fecal (53% as unchanged drug), kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
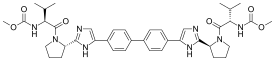
| Formula | C40H50N8O6 |
| Molar mass | 738.890 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Daclatasvir, sold under the brand name Daklinza, is an antiviral medication used in combination with other medications to treat hepatitis C (HCV).[5] The other medications used in combination include sofosbuvir, ribavirin, and interferon, vary depending on the virus type and whether the person has cirrhosis.[3] It is taken by mouth.[5]
Common side effects when used with sofusbivir and daclatasvir include headache, feeling tired, and nausea.[4] With daclatasvir, sofosbuvir, and ribavirin the most common side effects are headache, feeling tired, nausea, and red blood cell breakdown.[4] It should not be used with St. John's wort, rifampin, or carbamazepine.[5] It works by inhibiting the HCV protein NS5A.[3]
Daclatasvir was approved for use in the European Union in 2014, and the United States and India in 2015.[6] It is on the World Health Organization's List of Essential Medicines.[7]
The brand Daklinza is being withdrawn by Bristol Myers Squibb in countries where the drug is not typically prescribed, and Bristol Myers Squibb says it will not enforce its patents in those countries.[8]
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2015". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 10 April 2023.
- ^ "Health Canada New Drug Authorizations: 2015 Highlights". Health Canada. 4 May 2016. Retrieved 7 April 2024.
- ^ a b c "Daklinza film-coated tablets – Summary of Product Characteristics (SPC) - (eMC)". Electronic Medicines Compendium. September 2016. Archived from the original on 2016-11-09.
- ^ a b c d e "Daklinza (daclatasvir) tablets, for oral useInitial U.S. Approval: 2015". DailyMed. 16 October 2019. Retrieved 19 November 2021.
- ^ a b c "Daclatasvir Dihydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 5 November 2016. Retrieved 6 December 2016.
- ^ "Hepatitis C Treatment Snapshots: Daclatasvir" (PDF). amFAR TreatAsia. February 2016. Archived (PDF) from the original on 2016-09-03.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "Important information about the discontinuation of Daklinza". Bristol Myers Squibb. 9 March 2020. Retrieved 19 June 2020.