| |

| |
| Names | |
|---|---|
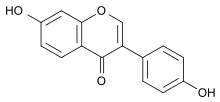
| IUPAC name
4′,7-Dihydroxyisoflavone
| |
| Systematic IUPAC name
7-Hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
7-Hydroxy-3-(4-hydroxyphenyl)chromen-4-one
Daidzeol Isoaurostatin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.942 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H10O4 | |
| Molar mass | 254.23 g/mol |
| Appearance | Pale yellow prisms |
| Melting point | 315 to 323 °C (599 to 613 °F; 588 to 596 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Daidzein (7-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one) is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones are produced in plants through the phenylpropanoid pathway of secondary metabolism and are used as signal carriers, and defense responses to pathogenic attacks.[2] In humans, recent research has shown the viability of using daidzein in medicine for menopausal relief, osteoporosis, blood cholesterol, and lowering the risk of some hormone-related cancers, and heart disease. Despite the known health benefits, the use of both puerarin and daidzein is limited by their poor bioavailability and low water solubility.[3]
- ^ Merck Index, 11th Edition, 2805.
- ^ Jung W.S.; Yu, O.; Lau, C., S.M.; O'Keefe, D.P.; Odell, J.; Fader, G.; McGonigle, B. (2000). "Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes". Nature Biotechnology. 18 (2): 208–212. doi:10.1038/72671. ISSN 1546-1696. PMID 10657130. S2CID 1717934.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wang Y.C.; Yang M.; Qin J.J.; Wa W.Q. (2022). "Interactions between puerarin/daidzein and micellar casein". Journal of Food Biochemistry. 46 (2): e14048. doi:10.1111/jfbc.14048. PMID 34981538. S2CID 245670986.