 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Danatrol, Danocrine, Danol, Danoval, others |
| Other names | WIN-17757; 2,3-Isoxazolethisterone; 2,3-Isoxazol-17α-ethynyltestosterone; 17α-Ethynyl-17β-hydroxyandrost-4-en-[2,3-d]isoxazole |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682599 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Androgen; Anabolic steroid; Progestogen; Progestin; Antigonadotropin; Steroidogenesis inhibitor; Antiestrogen |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Saturable with dosage, higher with food intake[2] |
| Protein binding | To albumin, SHBG, CBG[3][4][5] |
| Metabolism | Liver (CYP3A4)[9][6] |
| Metabolites | • 2-OHM-Ethisterone[6] • Ethisterone[7][8] |
| Elimination half-life | Acute: 3–10 hours[9][2] Chronic: 24–26 hours[9] |
| Excretion | Urine, feces[9][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.037.503 |
| Chemical and physical data | |
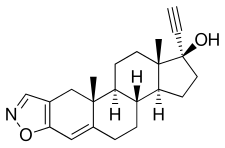
| Formula | C22H27NO2 |
| Molar mass | 337.463 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Danazol, sold as Danocrine and other brand names, is a medication used in the treatment of endometriosis, fibrocystic breast disease, hereditary angioedema and other conditions.[9][2][10][11][12] It is taken by mouth.[2]
The use of danazol is limited by masculinizing side effects such as acne, excessive hair growth, and voice deepening.[2][13] Danazol has a complex mechanism of action, and is characterized as a weak androgen and anabolic steroid, a weak progestogen, a weak antigonadotropin, a weak steroidogenesis inhibitor, and a functional antiestrogen.[5][14][15][16]
Danazol was discovered in 1963 and was introduced for medical use in 1971.[14][17][18][19] Due to their improved side-effect profiles, particularly their lack of masculinizing side effects, danazol has largely been replaced by gonadotropin-releasing hormone analogues (GnRH analogues) in the treatment of endometriosis.[4]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b c d e f "Danocrine Brand of Danazol Capsules, USP" (PDF). Sanofi-Aventis U.S. LLC. U.S. Food and Drug Administration.
- ^ Griffin JP, D'Arcy PF (17 November 1997). A Manual of Adverse Drug Interactions. Elsevier. pp. 194–. ISBN 978-0-08-052583-9.
- ^ a b Nieschlag E, Behre HM, Nieschlag S (13 January 2010). Andrology: Male Reproductive Health and Dysfunction. Springer Science & Business Media. pp. 426–428. ISBN 978-3-540-78355-8.
- ^ a b Cite error: The named reference
ThomasRock2012was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
LemkeWilliams2012was invoked but never defined (see the help page). - ^ Cite error: The named reference
Dörwald2013was invoked but never defined (see the help page). - ^ Cite error: The named reference
Kurman2013was invoked but never defined (see the help page). - ^ a b c d e Brayfield A, ed. (30 October 2013). "Danazol". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 1 April 2014.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 345–. ISBN 978-1-4757-2085-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 293–. ISBN 978-3-88763-075-1.
- ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 91–. ISBN 978-94-011-4439-1.
- ^ Selak V, Farquhar C, Prentice A, Singla A (October 2007). Farquhar C (ed.). "Danazol for pelvic pain associated with endometriosis". The Cochrane Database of Systematic Reviews (4): CD000068. doi:10.1002/14651858.CD000068.pub2. hdl:2292/28213. PMID 17943735.
- ^ a b Cite error: The named reference
JonesRock2015was invoked but never defined (see the help page). - ^ Cite error: The named reference
Lupulescu1990was invoked but never defined (see the help page). - ^ Cite error: The named reference
AltchekDeligdisch2003was invoked but never defined (see the help page). - ^ Cite error: The named reference
NeumannPotts1979was invoked but never defined (see the help page). - ^ Cite error: The named reference
EliasGwinup1983was invoked but never defined (see the help page). - ^ Cite error: The named reference
Dmowski1971was invoked but never defined (see the help page).