| |
| Names | |
|---|---|
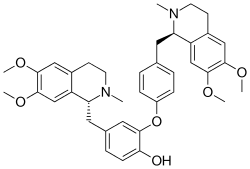
| Preferred IUPAC name
(11R,71R)-16,17,76,77-Tetramethoxy-12,72-dimethyl-11,12,13,14,71,72,73,74-octahydro-4-oxa-1,7(1)-diisoquinolina-3(1,3),5(1,4)-dibenzenaheptaphan-34-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.208.622 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C38H44N2O6 | |
| Molar mass | 624.778 g·mol−1 |
| Density | 1.186 g/mL |
| Melting point | 115 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dauricine is a plant metabolite, chemically classified as a phenol, an aromatic ether, and an isoquinoline alkaloid.[1] It has been isolated from the Asian vine Menispermum dauricum, commonly known as Asian moonseed, and the North American vine Menispermum canadense, commonly known as Canadian moonseed.[2] Scientists Tetsuji Kametani and Keiichiro Fukumoto of Japan are credited with being the first to synthesize dauricine in 1964, using both the Arndt-Eistert reaction and Bischler-Napieralski reaction to do so.[3] Dauricine has been studied in vitro for its potential to inhibit cancer cell growth[4][5][6][7] and to block cardiac transmembrane Na+, K+, and Ca2+ ion currents.[8]
- ^ "CHEBI:4331 - dauricine". ChEBI. Retrieved 30 May 2015.
- ^ Kametani, Tetsuji; Fukumoto, Keiichiro (1964). "Total synthesis of (±)-dauricine". Tetrahedron Letters. 5 (38): 2771–2775. doi:10.1016/S0040-4039(00)71728-X.
- ^ Manske, R.H.F. (1967). The Alkaloids: Chemistry and Physiology V9. New York: Academic Press. p. 141. ISBN 9780080865331. Retrieved 30 May 2015.
- ^ Yang, Zhengfeng; Li, Chenghai; Wang, Xiu; Zhai, Chunyan; Yi, Zhengfang; Wang, Lei; Liu, Bisheng; Du, Bing; Wu, Huihui; Guo, Xizhi; Liu, Mingyao; Li, Dali; Luo, Jian (2010). "Dauricine induces apoptosis, inhibits proliferation and invasion through inhibiting NF-kappaB signaling pathway in colon cancer cells". J. Cell. Physiol. 225 (1): 266–75. doi:10.1002/jcp.22261. PMID 20509140. S2CID 5501319.
- ^ Jin, Hua; Dai, Jieyu; Chen, Xiaoyan; Liu, Jia; Zhong, Dafang; Gu, Yansong; Zheng, Jiang (2009). "Pulmonary Toxicity and Metabolic Activation of Dauricine in CD-1 Mice". The Journal of Pharmacology and Experimental Therapeutics. 332 (3): 738–46. doi:10.1124/jpet.109.162297. PMID 20008063. S2CID 21824941.
- ^ Tang, Xu-dong; Zhou, Xin; Zhou, Ke-yuan (2009). "Dauricine inhibits insulin-like growth factor-I-induced hypoxia inducible factor 1alpha protein accumulation and vascular endothelial growth factor expression in human breast cancer cells". Acta Pharmacol Sin. 30 (5): 605–16. doi:10.1038/aps.2009.8. PMC 4002832. PMID 19349962.
- ^ Wang, Jun; Li, Yuan; Zu, Xiong-Bing; Chen, Min-Feng; Qi, Li (2012). "Dauricine can inhibit the activity of proliferation of urinary tract tumor cells". Asian Pac J Trop Med. 5 (12): 973–76. doi:10.1016/S1995-7645(12)60185-0. PMID 23199717.
- ^ Qian, JQ (2002). "Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives". Acta Pharmacol Sin. 23 (12): 1086–92. PMID 12466045.