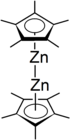| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C20H30Zn2 | |||
| Molar mass | 401.22 g·mol−1 | ||
| Appearance | colorless crystalline solid | ||
| Melting point | 110 °C (230 °F; 383 K) (decomposes) | ||
| Reacts | |||
| Solubility | soluble in diethyl ether, benzene, pentane, and tetrahydrofuran | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
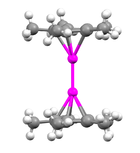
Decamethyldizincocene is an organozinc compound with the formula [Zn2(η5–C5Me5)2]. It is the first[1] and an unusual example of a compound with a Zn-Zn bond.[2] Decamethyldizincocene is a colorless crystalline solid that burns spontaneously in the presence of oxygen and reacts with water. It is stable at room temperature and especially soluble in diethyl ether, benzene, pentane, or tetrahydrofuran.[3]
- ^ Housecroft, C. E.; Sharpe, A. G. (2008). Inorganic Chemistry (3rd ed.). Prentice Hall. p. 843. ISBN 978-0-13-175553-6.
- ^ Resa, I.; Carmona, E.; Gutierrez-Puebla, E.; Monge, A. (2004). "Decamethyldizincocene, a Stable Compound of Zn(I) with a Zn-Zn Bond". Science. 305 (5687): 1136–8. Bibcode:2004Sci...305.1136R. doi:10.1126/science.1101356. PMID 15326350. S2CID 38990338.
- ^ Cite error: The named reference
GrirraneResawas invoked but never defined (see the help page).