 | |
| Clinical data | |
|---|---|
| Trade names | Dacogen, Demylocan |
| Other names | 5-aza-2'-deoxycytidine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608009 |
| License data |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | <1% |
| Elimination half-life | 30 minutes |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.017.355 |
| Chemical and physical data | |
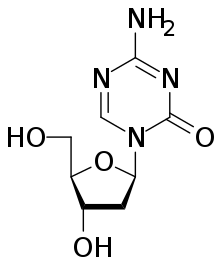
| Formula | C8H12N4O4 |
| Molar mass | 228.208 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Decitabine (i.e., 5-aza-2′-deoxycytidine), sold under the brand name Dacogen among others, acts as a nucleic acid synthesis inhibitor.[4] It is a medication for the treatment of myelodysplastic syndromes, a class of conditions where certain blood cells are dysfunctional, and for acute myeloid leukemia (AML).[5] Chemically, it is a cytidine analog.
- ^ "Summary Basis of Decision (SBD) for Dacogen". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ "Summary Basis of Decision (SBD) for Demylocan". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ "Dacogen EPAR". European Medicines Agency (EMA). 8 June 2006. Retrieved 4 June 2024.
- ^ "Decitabine". National Center for Biotechnology Information. Retrieved September 24, 2016.
- ^ "EC Approves Marketing Authorization Of DACOGEN For Acute Myeloid Leukemia". 2012-09-28. Retrieved 28 September 2012.