| |
| Names | |
|---|---|
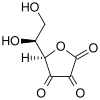
| IUPAC name
L-threo-Hexo-2,3-diulosono-1,4-lactone
| |
| Systematic IUPAC name
(5R)-5-[(1S)-1,2-Dihydroxyethyl]oxolane-2,3,4-trione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.019 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H6O6 | |
| Molar mass | 174.108 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dehydroascorbic acid (DHA) is an oxidized form of ascorbic acid (vitamin C). It is actively imported into the endoplasmic reticulum of cells via glucose transporters.[1] It is trapped therein by reduction back to ascorbic acid by glutathione and other thiols.[2] The (free) chemical radical semidehydroascorbic acid (SDA) also belongs to the group of oxidized ascorbic acids.
- ^ May, J. M. (1998). "Ascorbate function and metabolism in the human erythrocyte". Frontiers in Bioscience. 3 (4): d1–10. doi:10.2741/a262. PMID 9405334.
- ^ Welch, R. W.; Wang, Y.; Crossman, A. Jr.; Park, J. B.; Kirk, K. L.; Levine, M. (1995). "Accumulation of Vitamin C (Ascorbate) and Its Oxidized Metabolite Dehydroascorbic Acid Occurs by Separate Mechanisms". Journal of Biological Chemistry. 270 (21): 12584–12592. doi:10.1074/jbc.270.21.12584. PMID 7759506.