 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Rescriptor |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a600034 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Protein binding | 98% |
| Metabolism | Liver (CYP3A4- and CYP2D6-mediated) |
| Elimination half-life | 5.8 hours |
| Excretion | Kidney (51%) and feces (44%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
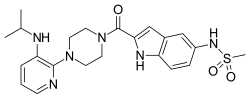
| Formula | C22H28N6O3S |
| Molar mass | 456.57 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Delavirdine (DLV) (brand name Rescriptor) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) marketed by ViiV Healthcare. It is used as part of highly active antiretroviral therapy (HAART) for the treatment of human immunodeficiency virus (HIV) type 1. It is presented as the mesylate. The recommended dosage is 400 mg, three times a day.
Although delavirdine was approved by the U.S. Food and Drug Administration in 1997, its efficacy is lower than other NNRTIs, especially efavirenz, and it also has an inconvenient schedule. These factors have led the U.S. DHHS not to recommend its use as part of initial therapy.[1] The risk of cross-resistance across the NNRTI class, as well as its complex set of drug interactions, makes the place of delavirdine in second-line and salvage therapy unclear, and it is currently rarely used.
Its manufacturing and distribution was discontinued in the United States and Canada.[2][3][4]
- ^ DHHS panel. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents (May 4, 2006). (Available for download from AIDSInfo Archived 2006-05-06 at the Wayback Machine)
- ^ RPh, Diana Ernst (11 May 2017). "HIV Drug Soon to Be Unavailable". MPR.
- ^ "Delavirdine: Indications, Side Effects, Warnings". Drugs.com.
- ^ "Prescription drug RESCRIPTOR (delavirdine mesylate) – DrugFinder.ca". drugfinder.ca.