 | |
 | |
| Clinical data | |
|---|---|
| Other names | Colcemid |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.832 |
| Chemical and physical data | |
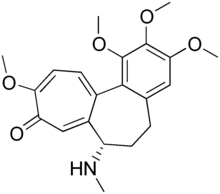
| Formula | C21H25NO5 |
| Molar mass | 371.433 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Demecolcine (INN; also known as colcemid) is a drug used in chemotherapy. It is closely related to the natural alkaloid colchicine with the replacement of the acetyl group on the amino moiety with methyl, but it is less toxic. It depolymerises microtubules and limits microtubule formation (inactivates spindle fibre formation), thus arresting cells in metaphase and allowing cell harvest and karyotyping to be performed.
During cell division, demecolcine inhibits mitosis at metaphase by inhibiting spindle formation. Medically, demecolcine has been used to improve the results of cancer radiotherapy by synchronising tumour cells at metaphase, the radiosensitive stage of the cell cycle.[1]
In animal cloning procedures, demecolcine makes an ovum eject its nucleus, creating space for insertion of a new nucleus.[2]
- ^ Sutton M (February 1965). "Superior Mediastinal Obstruction Treated with Demecolcine Followed by Radiotherapy". British Medical Journal. 1 (5433): 495–6. doi:10.1136/bmj.1.5433.495. PMC 2165889. PMID 14238680.
- ^ Hou J, Lei T, Liu L, Cui X, An X, Chen Y (2006). "Demecolcine-induced enucleation of sheep meiotically maturing oocytes". Reproduction, Nutrition, Development. 46 (2): 219–26. doi:10.1051/rnd:2006002. PMID 16597428.