 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Norpramin, Pertofrane, others |
| Other names | Desmethylimipramine; Norimipramine; EX-4355; G-35020; JB-8181; NSC-114901[1][2][3] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682387 |
| Routes of administration | Oral, intramuscular injection |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60–70%[6] |
| Protein binding | 91%[6] |
| Metabolism | Liver (CYP2D6)[7] |
| Elimination half-life | 12–30 hours[6] |
| Excretion | Urine (70%), feces[6] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.037 |
| Chemical and physical data | |
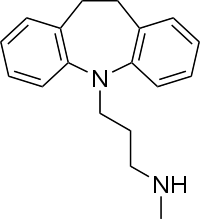
| Formula | C18H22N2 |
| Molar mass | 266.388 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Desipramine, sold under the brand name Norpramin among others, is a tricyclic antidepressant (TCA) used in the treatment of depression.[8] It acts as a relatively selective norepinephrine reuptake inhibitor, though it does also have other activities such as weak serotonin reuptake inhibitory, α1-blocking, antihistamine, and anticholinergic effects. The drug is not considered a first-line treatment for depression since the introduction of selective serotonin reuptake inhibitor (SSRI) antidepressants, which have fewer side effects and are safer in overdose.
- ^ Cite error: The named reference
Elks2014was invoked but never defined (see the help page). - ^ Cite error: The named reference
IndexNominum2000was invoked but never defined (see the help page). - ^ Cite error: The named reference
Drugs.comwas invoked but never defined (see the help page). - ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ a b c d Lemke TL, Williams DA (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 588–. ISBN 978-1-60913-345-0.
- ^ Sallee FR, Pollock BG (May 1990). "Clinical pharmacokinetics of imipramine and desipramine". Clinical Pharmacokinetics. 18 (5): 346–364. doi:10.2165/00003088-199018050-00002. PMID 2185906. S2CID 37529573.
- ^ Brunton L, Chabner B, Knollman B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill Professional. ISBN 978-0-07-162442-8.