 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Clarinex, Aerius, Allex, others[1] |
| Other names | descarboethoxyloratadine[2] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602002 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Rapidly absorbed |
| Protein binding | 83 to 87% |
| Metabolism | UGT2B10, CYP2C8 |
| Metabolites | 3-Hydroxydesloratadine |
| Onset of action | within 1 hour[6] |
| Elimination half-life | 27 hours,[6] 33.7 hours in elderly patients[3] |
| Duration of action | up to 24 hours[6] |
| Excretion | 40% as conjugated metabolites into urine Similar amount into the feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.166.554 |
| Chemical and physical data | |
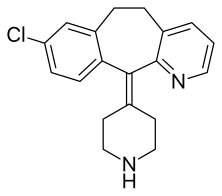
| Formula | C19H19ClN2 |
| Molar mass | 310.83 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Desloratadine. sold under the brand name Clarinex among others, is a tricyclic H1 inverse agonist that is used to treat allergies. It is an active metabolite of loratadine.[6]
It was patented in 1984 and came into medical use in 2001.[7] It was brought to the market in the US by Schering Corporation, later named Schering-Plough.[3]
- ^ Murdoch D, Goa KL, Keam SJ (7 April 2003). "Desloratadine: an update of its efficacy in the management of allergic disorders". Drugs. 63 (19): 2051–2077. doi:10.2165/00003495-200363190-00010. PMID 12962522. S2CID 195689362.
- ^ Schering Corporation (2000). "CLARITIN brand of Loratadine - Full Prescribing Information (US FDA)" (PDF). US FDA. Retrieved 17 May 2024.
loratadine is metabolized to descarboethoxyloratadine predominantly by cytochrome P450 3A4 (CYP3A4) and, to a lesser extent, by cytochrome P450 2D6 (CYP2D6).
- ^ a b c "Clarinex- desloratadine tablet, film coated". DailyMed. 14 November 2022. Retrieved 18 May 2024.
- ^ "Clarinex-D 12 HOUR- desloratadine and pseudoephedrine sulfate tablet, extended release". DailyMed. 14 November 2022. Retrieved 18 May 2024.
- ^ "Allex EPAR". European Medicines Agency (EMA). 19 May 2004.
- ^ a b c d Lieberman P, Hernandez-Trujillo V, Lieberman J, Frew AJ (2008). "Antihistamines". Clinical Immunology. Elsevier. pp. 1317–1329. doi:10.1016/b978-0-323-04404-2.10089-2. ISBN 9780323044042.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 549. ISBN 9783527607495.