| |
| |
| Names | |
|---|---|
| IUPAC name
Diazomethane
| |
| Other names
Azimethylene,
Azomethylene, | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.803 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH2N2 | |
| Molar mass | 42.04 g/mol |
| Appearance | Yellow gas |
| Odor | musty |
| Density | 1.4 (air=1) |
| Melting point | −145 °C (−229 °F; 128 K) |
| Boiling point | −23 °C (−9 °F; 250 K) |
| hydrolysis[1] | |
| Conjugate acid | Methyldiazonium |
| Structure | |
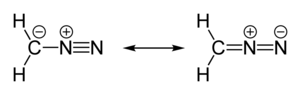
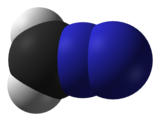
| linear C=N=N | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic and explosive |
| GHS labelling: | |
 
| |
| Danger | |
| H350 | |
| P201, P202, P281, P308+P313, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
175 ppm (cat, 10 min)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.2 ppm (0.4 mg/m3)[2] |
REL (Recommended)
|
TWA 0.2 ppm (0.4 mg/m3)[2] |
IDLH (Immediate danger)
|
2 ppm[2] |
| Related compounds | |
Related functional groups;
compounds |
R-N=N=N (azide), R-N=N-R (azo); R2CN2 R = Ph, tms, CF3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions.[4] Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane.[5]
- ^ ICSC 1256 – DIAZOMETHANE
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0182". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Diazomethane". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Proctor, Lee D.; Warr, Antony J. (November 2002). "Development of a Continuous Process for the Industrial Generation of Diazomethane". Organic Process Research & Development. 6 (6): 884–892. doi:10.1021/op020049k.
- ^ Shioiri, Takayuki; Aoyama, Toyohiko; Snowden, Timothy (2001). "Trimethylsilyldiazomethane". Encyclopedia of Reagents for Organic Synthesis. e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt298.pub2. ISBN 0471936235.
