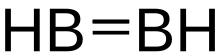
| |

| |
| Names | |
|---|---|
| IUPAC name
Diborene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| B2H2 | |
| Molar mass | 23.64 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diborane(2), also known as diborene, is an inorganic compound with the formula B2H2. The number 2 in diborane(2) indicates the number of hydrogen atoms bonded to the boron complex. There are other forms of diborane with different numbers of hydrogen atoms, including diborane(4) and diborane(6).
Diborane(2) is a highly reactive molecule that rapidly decomposes, making it a challenge to study experimentally under ambient conditions. To observe diborane(2) experimentally, high-vacuum and low temperature conditions using matrix isolation techniques are required, such as trapping the molecule in inert matrices like neon or argon.[1][2] As a result of these difficult synthesis conditions, its properties and behaviour have been predominantly studied using theoretical models and computational simulations.
Diborene also refers to a series of molecules with the formula R:(BH)=(BH):R or R-B=B-R where R is an organic group.[3][4] Diborene derivatives are relatively stable and can be stored at room temperature under inert conditions. They have been synthesized and characterized experimentally, and have shown potential in a variety of applications.
- ^ Tague, Thomas J.; Andrews, Lester (1994). "Reactions of Pulsed-Laser Evaporated Boron Atoms with Hydrogen. Infrared Spectra of Boron Hydride Intermediate Species in Solid Argon". Journal of the American Chemical Society. 116 (11): 4970–4976. doi:10.1021/ja00090a048. ISSN 0002-7863.
- ^ Knight, Lon B.; Kerr, Kelly; Miller, P. K.; Arrington, C. A. (1995). "ESR Investigation of the HBBH(X3.SIGMA.) Radical in Neon and Argon Matrixes at 4 K. Comparison with ab Initio SCF and CI Calculations". The Journal of Physical Chemistry. 99 (46): 16842–16848. doi:10.1021/j100046a009. ISSN 0022-3654.
- ^ Wang, Yuzhong; Quillian, Brandon; Wei, Pingrong; Wannere, Chaitanya S.; Xie, Yaoming; King, R. Bruce; Schaefer, Henry F.; Schleyer, Paul v. R.; Robinson, Gregory H. (2007-10-01). "A Stable Neutral Diborene Containing a BB Double Bond". Journal of the American Chemical Society. 129 (41): 12412–12413. doi:10.1021/ja075932i. ISSN 0002-7863. PMID 17887683.
- ^ Borthakur, Rosmita; Saha, Koushik; Kar, Sourav; Ghosh, Sundargopal (2019-11-15). "Recent advances in transition metal diborane(6), diborane(4) and diborene(2) chemistry". Coordination Chemistry Reviews. 399: 213021. doi:10.1016/j.ccr.2019.213021. ISSN 0010-8545. S2CID 202880767.