
| |
| |
| Names | |
|---|---|
| IUPAC name
Dichlorine heptoxide
| |
| Other names
Chlorine(VII) oxide; Perchloric anhydride; (Perchloryloxy)chlorane trioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
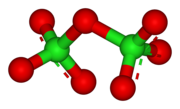
| Cl2O7 | |
| Molar mass | 182.901 g/mol |
| Appearance | colorless liquid, colorless gas |
| Density | 1.9 g/cm3 |
| Melting point | −91.57 °C (−132.83 °F; 181.58 K) |
| Boiling point | 82.07 °C (179.73 °F; 355.22 K) |
| hydrolyzes to form perchloric acid | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
275.7 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
oxidizer, contact explosive[1] |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
Manganese heptoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dichlorine heptoxide is the chemical compound with the formula Cl2O7. This chlorine oxide is the anhydride of perchloric acid. It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pentoxide:[1]
- 2 HClO4 + P4O10 → Cl2O7 + H2P4O11
The chlorine(VII) oxide can be distilled off from the mixture.
It may also be formed by illumination of mixtures of chlorine and ozone with blue light.[2] It slowly hydrolyzes back to perchloric acid.
- ^ a b Holleman, Arnold F.; Wiberg, Egon (2001). Inorganic chemistry. Translated by Mary Eagleson; William Brewer. San Diego: Academic Press. p. 464. ISBN 0-12-352651-5.
- ^ Byrns, A. C.; Rollefson, G. K. (1934). "The Formation of Chlorine Heptoxide on Illumination of Mixtures of Chlorine and Ozone". Journal of the American Chemical Society. 56 (5): 1250–1251. doi:10.1021/ja01320a506.
