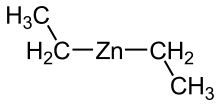
| |
| |
| Names | |
|---|---|
| IUPAC name
diethylzinc
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.330 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1366 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| (C2H5)2Zn | |
| Molar mass | 123.50 g/mol |
| Density | 1.205 g/mL |
| Melting point | −28 °C (−18 °F; 245 K) |
| Boiling point | 117 °C (243 °F; 390 K) |
| Reacts | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable and corrosive liquid, pyrophoric in air, may explode in contact with water. |
| GHS labelling: | |
  
| |
| Danger | |
| H225, H250, H260, H302+H312+H332, H314, H410 | |
| P210, P222, P223, P231+P232, P233, P240, P241, P242, P243, P260, P264, P273, P280, P301+P330+P331, P302+P334, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P335+P334, P363, P370+P378, P391, P402+P404, P403+P235, P405, P422, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diethylzinc (C2H5)2Zn, or DEZ, is a highly pyrophoric and reactive organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry. It is available commercially as a solution in hexanes, heptane, or toluene, or as a pure liquid.
