| |
| Names | |
|---|---|
| Preferred IUPAC name
Dihydroxypropanedioic acid | |
| Other names
Mesoxalic acid monohydrate
Oxomalonic acid monohydrate Ketomalonic acid monohydrate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.372 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H4O6 | |
| Molar mass | 136.059 g·mol−1 |
| Melting point | 119 to 120 °C (246 to 248 °F; 392 to 393 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
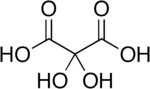
Dihydroxymalonic acid is an organic compound with formula C3H4O6 or HO-(C=O)-C(OH)2-(C=O)-OH, found in some plants such as alfalfa and in beet molasses.[2]
The compound is also called dihydroxymesoxalic acid and dihydroxypropanedioic acid. It can be viewed as a hydrate derivative of mesoxalic acid, and is often called mesoxalic acid monohydrate and similar names.[3] This compound is unusual in containing stable geminal hydroxy groups.
Dihydroxymalonic acid is a water-soluble white solid. It crystallizes in deliquescent prisms that melt between 113 °C and 121 °C without loss of water.[4] It has been used in medical research as a hypoglycemic agent[5] and was patented in the United States in 1997 as a fast-acting antidote to cyanide poisoning.[6]
- ^ Merck Index, 12th Edition, 5971.
- ^ Deichsel, Theodor (1864). "Ueber die Mesoxalsäure". J. Prakt. Chem. (in German). 93 (1): 193–208. doi:10.1002/prac.18640930139.
- ^ E. T. Urbansky, W. J. Bashe (2000). Journal of Chromatography A, volume 867, pp. 143–149.
- ^ Cite error: The named reference
roscoewas invoked but never defined (see the help page). - ^ Yoshito KOBAYASHI, Shigeru OHASHI, Shinzaburo TANAKA and Akitoshi SHIOYA (1955), Hypoglycemic Action of Sodium Mesoxalate with Special Reference to Hyperfunction of Pituitary-Adrenal Cortical System in Dogs Exposed to Cold[permanent dead link]. Proceedings of the Japan Academy, volume 31, issue 8, pp.493–497.
- ^ "Method for the treatment of cyanide poisoning". Archived from the original on 2017-05-11. Retrieved 2009-11-13.