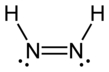| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Diazene
| |||
| Other names
Diimide
Diimine Dihydridodinitrogen Azodihydrogen | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| KEGG | |||
| MeSH | Diazene | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| H2N2 | |||
| Molar mass | 30.030 g·mol−1 | ||
| Appearance | Yellow gas | ||
| Melting point | −80 °C (−112 °F; 193 K) | ||
| Related compounds | |||
Other anions
|
diphosphene dinitrogen difluoride | ||
Other cations
|
azo compounds | ||
Related Binary azanes
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
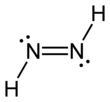
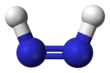
Diimide, also called diazene or diimine, is a compound having the formula HN=NH. It exists as two geometric isomers, E (trans) and Z (cis). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an example of an organic diazene.