 | |
| Combination of | |
|---|---|
| Diphenhydramine | Antihistamine, sedative |
| 8-chlorotheophylline | Stimulant |
| Clinical data | |
| Trade names | Dramamine, Draminate, Gravol, others |
| Other names | Diphenhydramine/8-chlorotheophylline salt |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607046 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, rectal, intravascular, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 5.5 hours[1] (diphenhydramine component) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.593 |
| | |
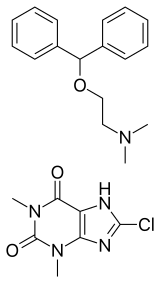
Dimenhydrinate, also known as diphenhydramine/8-chlorotheophylline salt and sold under the brand name Dramamine, Gravol, among others, is an over-the-counter medication used to treat motion sickness and nausea. Dimenhydrinate is a theoclate salt composed of diphenhydramine and 8-chlorotheophylline (a theophylline relative) in a 1:1 ratio.[2]
Dimenhydrinate was introduced to the market by G.D. Searle in 1949.[3][4]
- ^ Scavone JM, Luna BG, Harmatz JS, von Moltke L, Greenblatt DJ (April 1990). "Diphenhydramine kinetics following intravenous, oral, and sublingual dimenhydrinate administration". Biopharmaceutics & Drug Disposition. 11 (3): 185–189. doi:10.1002/bdd.2510110302. PMID 2328304.
- ^ Zabirowicz ES, Gan TJ (2019). "34 - Pharmacology of Postoperative Nausea and Vomiting". In Hemmings Jr HC, Talmage ED (eds.). Pharmacology and Physiology for Anesthesia (Second ed.). Elsevier Inc. pp. 671–692. doi:10.1016/B978-0-323-48110-6.00034-X. ISBN 978-0-323-48110-6. S2CID 81387334.
- ^ Newman A (21 June 2012). "New Dramamine Ads Take Aim at Summer Vacationers". The New York Times. Retrieved 26 June 2023.
- ^ Grauer N (12 February 2019). "Hopkins History Moments: Neil A. Grauer explains how Hopkins expertise helped prevent seasickness". Johns Hopkins Medicine. Retrieved 27 June 2023.