| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Dimethyl sulfate | |
| Other names
Dimethyl sulphate; Sulfuric acid dimethyl ester; Me2SO4; DMSO4; Dimethyl ester of sulfuric acid; Methyl sulfate, di-
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.963 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H6O4S | |
| Molar mass | 126.13 g/mol |
| Appearance | Colorless, oily liquid |
| Odor | faint, onion-like[1] |
| Density | 1.33 g/ml, liquid |
| Melting point | −32 °C (−26 °F; 241 K) |
| Boiling point | 188 °C (370 °F; 461 K) (decomposes) |
| Reacts | |
| Solubility | Methanol, dichloromethane, acetone |
| Vapor pressure | 0.1 mmHg (20 °C)[1] |
| −62.2×10−6 cm3/mol | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Extremely toxic, contact hazard, inhalation hazard, corrosive, environmental hazard, carcinogenic, mutagenic |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H314, H317, H330, H335, H341, H350 | |
| NFPA 704 (fire diamond) | |
| Flash point | 83 °C; 182 °F; 356 K[1] |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
8.6 ppm (rat, 4 hr) 75 ppm (guinea pig, 20 min) 53 ppm (mouse) 32 ppm (guinea pig, 1 hr)[2] |
LCLo (lowest published)
|
97 ppm (human, 10 min)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 ppm (5 mg/m3) [skin][1] |
REL (Recommended)
|
Ca TWA 0.1 ppm (0.5 mg/m3) [skin][1] |
IDLH (Immediate danger)
|
Ca [7 ppm][1] |
| Related compounds | |
Related compounds
|
Diethyl sulfate, methyl triflate, dimethyl carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
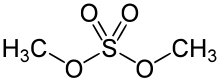
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as (CH3)2SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis. Me2SO4 is a colourless oily liquid with a slight onion-like odour. Like all strong alkylating agents, Me2SO4 is toxic.[3] Its use as a laboratory reagent has been superseded to some extent by methyl triflate, CF3SO3CH3, the methyl ester of trifluoromethanesulfonic acid.
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0229". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Dimethyl sulfate". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Weisenberger, Karl; Mayer, Dieter; Sandler, Stanley R. (2000). "Dialkyl Sulfates and Alkylsulfuric Acids". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a08_493. ISBN 978-3-527-30385-4.
