| |||
| |||
 A sample of dimethyl sulfoxide
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(Methanesulfinyl)methane | |||
| Systematic IUPAC name
(Methanesulfinyl)methane (substitutive) Dimethyl(oxido)sulfur (additive) | |||
| Other names
Methylsulfinylmethane
Methyl sulfoxide (2:1), Dermasorb[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | DMSO, Me2SO | ||
| 506008 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.604 | ||
| EC Number |
| ||
| 1556 | |||
| KEGG | |||
| MeSH | Dimethyl+sulfoxide | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H6OS | |||
| Molar mass | 78.13 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Density | 1.1004 g⋅cm−3 | ||
| Melting point | 19 °C (66 °F; 292 K) | ||
| Boiling point | 189 °C (372 °F; 462 K) | ||
| Miscible | |||
| Solubility in Diethyl ether | Not soluble | ||
| Vapor pressure | 0.556 millibars or 0.0556 kPa at 20 °C[2] | ||
| Acidity (pKa) | 35[3] | ||
Refractive index (nD)
|
1.479 εr = 48 | ||
| Viscosity | 1.996 cP at 20 °C | ||
| Structure | |||
| Cs | |||
| Trigonal pyramidal | |||
| 3.96 D | |||
| Pharmacology | |||
| G04BX13 (WHO) M02AX03 (WHO) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Irritant | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 89 °C (192 °F; 362 K) | ||
| Safety data sheet (SDS) | Oxford MSDS | ||
| Related compounds | |||
Related sulfoxides
|
Diethyl sulfoxide | ||
Related compounds
|
|||
| Supplementary data page | |||
| Dimethyl sulfoxide (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula (CH3)2SO. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO is metabolised to compounds that leave a garlic-like taste in the mouth after DMSO is absorbed by skin.[5]
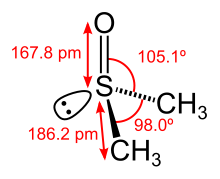
In terms of chemical structure, the molecule has idealized Cs symmetry. It has a trigonal pyramidal molecular geometry consistent with other three-coordinate S(IV) compounds,[6] with a nonbonded electron pair on the approximately tetrahedral sulfur atom.
- ^ DMSO (medication)
- ^ "Dimethyl Sulfoxide (DMSO) -- Technical". Atofina Chemicals, inc. Retrieved 26 May 2007.
- ^ Matthews WS, Bares JE, Bartmess JE, Bordwell FG, Cornforth FJ, Drucker GE, Margolin Z, McCallum RJ, McCollum GJ, Vanier NR (1975). "Equilibrium acidities of carbon acids. VI. Establishment of an absolute scale of acidities in dimethyl sulfoxide solution". J. Am. Chem. Soc. 97 (24): 7006–7014. doi:10.1021/ja00857a010.
- ^ "Dimethyl sulfoxide". pubchem.ncbi.nlm.nih.gov.
- ^ Novak KM, ed. (2002). Drug Facts and Comparisons (56th ed.). St. Louis, Missouri: Wolters Kluwer Health. p. 2345. ISBN 978-1-57439-110-7.
- ^ Thomas R, Shoemaker CB, Eriks K (1966). "The Molecular and Crystal Structure of Dimethyl Sulfoxide, (H3C)2SO". Acta Crystallogr. 21 (1): 12–20. Bibcode:1966AcCry..21...12T. doi:10.1107/S0365110X66002263.


