
| |
| |
 | |
| Names | |
|---|---|
| IUPAC name
3,3-Dimethyldioxirane
| |
| Other names
DMDO
Monoperoxyacetone, Murray's reagent | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
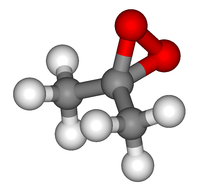
| C3H6O2 | |
| Molar mass | 74.08 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dimethyldioxirane (DMDO) is the organic compound with the formula (CH3)2CO2.[1][2] It is the dioxirane derived from acetone and can be considered as a monomer of acetone peroxide. It is a powerful selective oxidizing agent that finds some use in organic synthesis. It is known only in the form of a dilute solution, usually in acetone, and hence the properties of the pure material are largely unknown.[3]
- ^ "Robert W. Murray Biography". University of Missouri–St. Louis. Retrieved 14 October 2015.
- ^ Murray, Robert W. (July 1989). "Chemistry of dioxiranes. 12. Dioxiranes". Chemical Reviews. 89 (5): 1187–1201. doi:10.1021/cr00095a013.
- ^ Crandall, J. K.; Curc, R; D'Accolti, L; Fusco, C (15 Oct 2005). "Dimethyldioxirane". E-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd329.pub2. ISBN 0471936235.