| |

| |
| Names | |
|---|---|
| IUPAC name
N-Oxonitramide[1]
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.031.013 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2421 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| N2O3 | |
| Molar mass | 76.011 g·mol−1 |
| Appearance | Deep blue liquid |
| Density |
|
| Melting point | −100.7[2] °C (−149.3 °F; 172.5 K) |
| Boiling point | 3.5 °C (38.3 °F; 276.6 K) (dissociates[2]) |
| reacts to form nitrous acid | |
| Solubility | soluble in ether |
| −16.0·10−6 cm3/mol | |
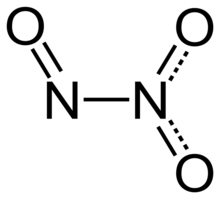
| Structure | |
| planar, Cs | |
| 2.122 D | |
| Thermochemistry | |
Heat capacity (C)
|
65.3 J/(mol·K) |
Std molar
entropy (S⦵298) |
314.63 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
91.20 kJ/mol |
| Hazards | |
| GHS labelling:[3] | |
  
| |
| Danger | |
| H270, H310+H330, H314 | |
| P220, P244, P260, P262, P264, P270, P271, P280, P284, P301+P330+P331, P302+P350, P303+P361+P353, P304+P340, P305+P351+P338, P310, P320, P321, P322, P361, P363, P370+P376, P403, P403+P233, P405, P410+P403, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dinitrogen trioxide (also known as nitrous anhydride) is the inorganic compound with the formula N2O3. It is a nitrogen oxide. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):[4]
- •
NO + •
NO
2 ⇌ N
2O
3
Dinitrogen trioxide is only isolable at low temperatures (i.e., in the liquid and solid phases). In liquid and solid states, it has a deep blue color.[2] At higher temperatures the equilibrium favors the constituent gases, with KD = 193 kPa (25 °C).[5][clarification needed]
This compound is sometimes called "nitrogen trioxide", but this name properly refers to another compound, the (uncharged) nitrate radical •NO3.
- ^ "Dinitrogen trioxide".
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 444. ISBN 978-0-08-037941-8.
- ^ "Dinitrogen trioxide". pubchem.ncbi.nlm.nih.gov. Retrieved 23 December 2021.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 521–22. ISBN 978-0-08-022057-4.
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
