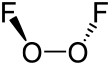| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Dioxygen difluoride | |||
| Systematic IUPAC name
Fluorooxygen hypofluorite | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | FOOF | ||
| ChEBI | |||
| ChemSpider | |||
| 1570 | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| O 2F 2 | |||
| Molar mass | 69.996 g·mol−1 | ||
| Appearance | orange as a solid red as a liquid | ||
| Density | 1.45 g/cm3 (at b.p.) | ||
| Melting point | −154 °C (−245 °F; 119 K) | ||
| Boiling point | −57 °C (−71 °F; 216 K) extrapolated | ||
| Solubility in other solvents | decomposes | ||
| Thermochemistry | |||
Heat capacity (C)
|
62.1 J/(mol·K) | ||
Std molar
entropy (S⦵298) |
277.2 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
19.2 kJ/mol | ||
Gibbs free energy (ΔfG⦵)
|
58.2 kJ/mol | ||
| Related compounds | |||
Related compounds
|
|||
| Hazards | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Dioxygen difluoride is a compound of fluorine and oxygen with the molecular formula O2F2. It can exist as an orange-red colored solid which melts into a red liquid at −163 °C (110 K). It is an extremely strong oxidant and decomposes into oxygen and fluorine even at −160 °C (113 K) at a rate of 4% per day — its lifetime at room temperature is thus extremely short.[1] Dioxygen difluoride reacts vigorously with nearly every chemical it encounters (including ordinary ice) leading to its onomatopoeic nickname FOOF (a play on its chemical structure and its explosive tendencies).[2]
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. Academic Press. ISBN 978-0-12-352651-9.
- ^ Lowe, Derek (2010-02-23). "Things I Won't Work With: Dioxygen Difluoride". www.science.org. Retrieved 2022-05-26.